Dronabinol Is a Safe Long-Term Treatment Option for Neuropathic Pain Patients
- PMID: 29073592
- PMCID: PMC5804828
- DOI: 10.1159/000481089
Dronabinol Is a Safe Long-Term Treatment Option for Neuropathic Pain Patients
Abstract
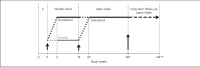
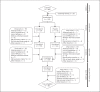
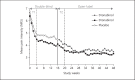
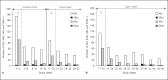
Treatment of neuropathic pain (NP) symptoms associated with multiple sclerosis (MS) is frequently insufficient. Yet, cannabis is still rarely offered for treatment of pain. This clinical trial aimed at showing the positive benefit-risk ratio of dronabinol. Two hundred forty MS patients with central NP entered a 16-weeks placebo-controlled phase-III study followed by a 32-weeks open-label period. One hundred patients continued therapy for overall up to 119 weeks. Primary endpoint was change of pain intensity on the 11-point Numerical Rating Scale over a 16-weeks treatment period. Safety was assessed on the basis of adverse reactions (ARs), signs of dependency and abuse. Pain intensity during 16-weeks dronabinol and placebo treatment was reduced by 1.92 and 1.81 points without significant difference in between (p = 0.676). Although the proportion of patients with ARs was higher under dronabinol compared to placebo (50.0 vs. 25.9%), it decreased during long-term use of dronabinol (26%). No signs of drug abuse and only one possible case of dependency occurred. The trial results demonstrate that dronabinol is a safe long-term treatment option.
Keywords: Clinical trial; Dronabinol; Long-term; Multiple sclerosis; Neuropathic pain; Safety; Tetrahydrocannabinol.
© 2017 The Author(s) Published by S. Karger AG, Basel.
Figures
References
-
- Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010–2014. Cannabinoids. 2016;11:1–18.
-
- Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpaa ML, Kent JL, Krane EJ, Lebel AA, Levy RM, Mackey SC, Mayer J, Miaskowski C, Raja SN, Rice AS, Schmader KE, Stacey B, Stanos S, Treede RD, Turk DC, Walco GA, Wells CD. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85((3 suppl)):S3–S14. - PMC - PubMed
-
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

