Viewpoints: how the hippocampus contributes to memory, navigation and cognition
- PMID: 29073641
- PMCID: PMC5943637
- DOI: 10.1038/nn.4661
Viewpoints: how the hippocampus contributes to memory, navigation and cognition
Erratum in
-
Publisher Correction: Viewpoints: how the hippocampus contributes to memory, navigation and cognition.Nat Neurosci. 2018 Jul;21(7):1018. doi: 10.1038/s41593-017-0034-8. Nat Neurosci. 2018. PMID: 29263406
Abstract
The hippocampus serves a critical function in memory, navigation, and cognition. Nature Neuroscience asked John Lisman to lead a group of researchers in a dialog on shared and distinct viewpoints on the hippocampus.
There has been a long history of studying the hippocampus, but recent work has made it possible to study the cellular and network basis of defined operations—operations that include cognitive processes that have been otherwise difficult to study (see Box 1 for useful terminology). These operations deal with the context-dependent representation of complex memories, the role of mental exploration based on imagined rather than real movements, and the use of recalled information for navigation and decision-making. The progress that has been made in understanding the hippocampus has motivated the study of other brain regions that provide hippocampal input or receive hippocampal output; the hippocampus is thus serving as a nucleating point for the larger goal of understanding the neural codes that allow inter-regional communication and more generally, understanding how memory-guided behavior is achieved by large scale integration of brain regions. In generating a discussion among experts in the study of the cognitive processes of the hippocampus, the editors and I have posed questions that probe important principles of hippocampal function. We hope that the resulting discussion will make clear to readers the progress that has been made, while also identifying issues where consensus has not yet been achieved and that should be pursued in future research. – John Lisman
Figures


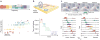

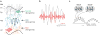
References
-
- Tulving E, Donaldson W, Bower GH. Organization of Memory. Academic Press; 1972.
-
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Clarendon Press; Oxford University Press; 1978.
-
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. MIT Press; 1993.
Publication types
MeSH terms
Grants and funding
- R01 MH095297/MH/NIMH NIH HHS/United States
- R01 NS074015/NS/NINDS NIH HHS/United States
- R01 MH110391/MH/NIMH NIH HHS/United States
- R01 MH052090/MH/NIMH NIH HHS/United States
- R01 MH080318/MH/NIMH NIH HHS/United States
- U01 NS090583/NS/NINDS NIH HHS/United States
- R01 MH112688/MH/NIMH NIH HHS/United States
- R01 MH051570/MH/NIMH NIH HHS/United States
- R01 DA043195/DA/NIDA NIH HHS/United States
- R01 MH054671/MH/NIMH NIH HHS/United States
- U01 NS090526/NS/NINDS NIH HHS/United States
- U19 NS104590/NS/NINDS NIH HHS/United States
- R56 MH080318/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

