A Systematic Review of the Effectiveness of Medical Cannabis for Psychiatric, Movement and Neurodegenerative Disorders
- PMID: 29073741
- PMCID: PMC5678490
- DOI: 10.9758/cpn.2017.15.4.301
A Systematic Review of the Effectiveness of Medical Cannabis for Psychiatric, Movement and Neurodegenerative Disorders
Abstract
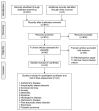
The discovery of endocannabinoid's role within the central nervous system and its potential therapeutic benefits have brought forth rising interest in the use of cannabis for medical purposes. The present review aimed to synthesize and evaluate the available evidences on the efficacy of cannabis and its derivatives for psychiatric, neurodegenerative and movement disorders. A systematic search of randomized controlled trials of cannabis and its derivatives were conducted via databases (PubMed, Embase and the Cochrane Central Register of Controlled Trials). A total of 24 reports that evaluated the use of medical cannabis for Alzheimer's disease, anorexia nervosa, anxiety, dementia, dystonia, Huntington's disease, Parkinson's disease, post-traumatic stress disorder (PTSD), psychosis and Tourette syndrome were included in this review. Trial quality was assessed with the Cochrane risk of bias tool. There is a lack of evidence on the therapeutic effects of cannabinoids for amyotrophic lateral sclerosis and dystonia. Although trials with positive findings were identified for anorexia nervosa, anxiety, PTSD, psychotic symptoms, agitation in Alzheimer's disease and dementia, Huntington's disease, and Tourette syndrome, and dyskinesia in Parkinson's disease, definitive conclusion on its efficacy could not be drawn. Evaluation of these low-quality trials, as rated on the Cochrane risk of bias tools, was challenged by methodological issues such as inadequate description of allocation concealment, blinding and underpowered sample size. More adequately powered controlled trials that examine the long and short term efficacy, safety and tolerability of cannabis for medical use, and the mechanisms underpinning the therapeutic potential are warranted.
Keywords: Cannabinoids; Cannabis; Mental disorders; Movement disorders; Neurodegenerative diseases.; Randomized controlled trial.
Figures
References
-
- Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556–1563. doi: 10.1212/WNL.0000000000000363. - DOI - PMC - PubMed
-
- Klumpers LE, Beumer TL, van Hasselt JG, Lipplaa A, Karger LB, Kleinloog HD, et al. Novel Δ(9) -tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamic effects. Br J Clin Pharmacol. 2012;74:42–53. doi: 10.1111/j.1365-2125.2012.04164.x. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources