Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial
- PMID: 29074098
- PMCID: PMC5777233
- DOI: 10.1016/S1470-2045(17)30680-0
Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial
Abstract
Background: Most patients with anaplastic lymphoma kinase (ALK)-rearranged or ROS proto-oncogene 1 (ROS1)-rearranged non-small-cell lung cancer (NSCLC) are sensitive to tyrosine kinase inhibitor (TKI) therapy, but resistance invariably develops, commonly within the CNS. This study aimed to analyse the safety, efficacy, and pharmacokinetic properties of lorlatinib, a novel, highly potent, selective, and brain-penetrant ALK and ROS1 TKI with preclinical activity against most known resistance mutations, in patients with advanced ALK-positive or ROS1-positive NSCLC.
Methods: In this international multicentre, open-label, single-arm, first-in-man phase 1 dose-escalation study, eligible patients had advanced ALK-positive or ROS1-positive NSCLC and were older than 18 years, with an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate end-organ function. Lorlatinib was administered orally to patients at doses ranging from 10 mg to 200 mg once daily or 35 mg to 100 mg twice daily, with a minimum of three patients receiving each dose. For some patients, tumour biopsy was done before lorlatinib treatment to identify ALK resistance mutations. Safety was assessed in patients who received at least one dose of lorlatinib; efficacy was assessed in the intention-to-treat population (patients who received at least one dose of study treatment and had either ALK or ROS1 rearrangement). The primary endpoint was dose-limiting toxicities during cycle 1 according to investigator assessment; secondary endpoints included safety, pharmacokinetics, and overall response. This study is ongoing and is registered with ClinicalTrials.gov, number NCT01970865.
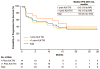
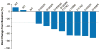
Findings: Between Jan 22, 2014, and July 10, 2015, 54 patients received at least one dose of lorlatinib, including 41 (77%) with ALK-positive and 12 (23%) with ROS1-positive NSCLC; one patient had unconfirmed ALK and ROS1 status. 28 (52%) patients had received two or more TKIs, and 39 (72%) patients had CNS metastases. The most common treatment-related adverse events among the 54 patients were hypercholesterolaemia (39 [72%] of 54 patients), hypertriglyceridaemia (21 [39%] of 54 patients), peripheral neuropathy (21 [39%] of 54 patients), and peripheral oedema (21 [39%] of 54 patients). One dose-limiting toxicity occurred at 200 mg (the patient did not take at least 16 of 21 prescribed total daily doses in cycle 1 because of toxicities attributable to study drug, which were grade 2 neurocognitive adverse events comprising slowed speech and mentation and word-finding difficulty). No maximum tolerated dose was identified. The recommended phase 2 dose was selected as 100 mg once daily. For ALK-positive patients, the proportion of patients who achieved an objective response was 19 (46%) of 41 patients (95% CI 31-63); for those who had received two or more TKIs, the proportion of patients with an objective response was 11 (42%) of 26 patients (23-63). In ROS1-positive patients, including seven crizotinib-pretreated patients, an objective response was achieved by six (50%) of 12 patients (95% CI 21-79).
Interpretation: In this phase 1, dose-escalation study, lorlatinib showed both systemic and intracranial activity in patients with advanced ALK-positive or ROS1-positive NSCLC, most of whom had CNS metastases and had previously had two or more TKI treatments fail. Therefore, lorlatinib might be an effective therapeutic strategy for patients with ALK-positive NSCLC who have become resistant to currently available TKIs, including second-generation ALK TKIs, and is being investigated in a phase 3 randomised controlled trial comparing lorlatinib to crizotinib (ClinicalTrials.gov, NCT03052608).
Funding: Pfizer.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
ATS has received fees for consulting/advisory board roles from Ariad, Blueprint Medicines, Daiichi Sankyo, EMD Serono, Genentech/Roche, Ignyta, KSQ, Loxo, Novartis, Pfizer, and Taiho, honoraria from Foundation Medicine, Novartis, Pfizer, and Genentech/Roche, and her institution has received research funding from Pfizer, Novartis, and Genentech/Roche. EF has received fees for consulting or advisory roles from Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Pfizer, and Genentech/Roche, and fees for serving on speaker bureaus from AstraZeneca, Bristol-Myers Squibb, and Novartis. TMB’s institution has received research funding from AbbVie, AstraZeneca, Calithera Biosciences, Daiichi Sankyo, Deciphera, Eli Lilly, Genentech/Roche, GlaxoSmithKline, Ignyta, ImmunoGen, Incyte, Kolltan Pharmaceuticals, Leap Therapeutics, MabVax, MedImmune, Medpacto Inc., Merck, Merrimack, Millennium, Mirati Therapeutics, Novartis, Peleton, Pfizer, Principia Biopharma, and Stemline Therapeutics. BB has received research funding from Pfizer. JFG has received personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, Genentech/Roche, Incyte, Loxo, Merck, Novartis, and Theravance, and travel expenses from Affymetrix. MJ reports that her institution has received research funding from AbbVie, Adaptimmune, Apexigen, Array BioPharma, AstraZeneca, BerGenBio, Checkpoint Therapeutics, Eli Lilly, EMD Serono, Genentech/Roche, Genmab, Janssen, Kadmon, Mirati Therapeutics, Merrimack, Novartis, OncoMed, Pfizer, Regeneron, Stemcentrix, and Tarveda, and fees for consulting/advisory board roles from Boehringer Ingelheim, Celgene, and Genentech/Roche. AA, JC, J-FM, and LPJ are employees of and own stock in Pfizer. JSC is an employee of InVentiv Health and works as a contractor for Pfizer. BJS has received fees for serving on advisory boards for and honoraria from AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Merck, Novartis, and Pfizer, and his institution has received clinical trial support from Pfizer. No other potential conflict of interest relevant to this article was reported.
Figures



Comment in
-
ALK and ROS1 rearrangement in NSCLC: rapidly evolving standards.Lancet Oncol. 2017 Dec;18(12):1555-1556. doi: 10.1016/S1470-2045(17)30708-8. Epub 2017 Oct 23. Lancet Oncol. 2017. PMID: 29074100 No abstract available.
-
Lorlatinib in ALK- and ROS1-positive NSCLC: the future has a start.Transl Lung Cancer Res. 2018 Apr;7(Suppl 2):S103-S106. doi: 10.21037/tlcr.2018.02.04. Transl Lung Cancer Res. 2018. PMID: 29782561 Free PMC article. No abstract available.
References
-
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. - PubMed
-
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. - PubMed
-
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. - PubMed

