High specific immune response to a bivalent anti-HPV vaccine in HIV-1-infected men in São Paulo, Brazil
- PMID: 29074177
- PMCID: PMC5886862
- DOI: 10.1016/j.pvr.2016.01.001
High specific immune response to a bivalent anti-HPV vaccine in HIV-1-infected men in São Paulo, Brazil
Abstract
Introduction: Infection with Human papillomavirus (HPV) has been reported as one of the most prevalent agent sexually transmitted diseases, but its true prevalence in men is not precisely known, mainly due to the near absence of symptoms. Moreover, few studies evaluating the post-vaccination immune response have been performed to date in men, hence the hypotheses tested in this study can be important to enable a better understanding of both the immunopathogenesis and the response to vaccination in HIV-infected patients, and to help in the elaboration of strategies of vaccination against HPV in the HIV-infected population.
Objectives: To analyze the specific response to antigens of HPV vaccine in HIV-infected men.
Methods: A total of 25 HIV-infected male patients who met the inclusion criteria during the data collection period were vaccinated; however, six (30%) had anti-HPV at baseline, and were not considered further in the analysis. Therefore, 19 HIV-infected individuals were included in the study, along with five healthy, HPV-seronegative controls.
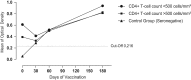
Results: Patients infected with HIV-1 were subdivided into two groups, A and B, according to their T CD4 cells count at the time of vaccination, namely: Group A: CD4>500; Group B: CD4<500. The proportion of seroconversion after immunization with three doses of a bivalent anti-HPV vaccine was 92%.
Conclusion: HIV-infected patients as well as HIV negative controls responded to anti-HPV vaccination, regardless of their T CD4 cells count and HIV plasma viral load. These results demonstrate that anti-HPV immunization in HIV-infected males is effective and should be encouraged, thus helping to decrease the risk of infection, mortality and morbidity of diseases associated with HPV in men.
Keywords: HIV-1; HPV; Immune response; Vaccine.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- zur Hausen H., de Villiers E.M., Gissmann L. Papillomavirus infections and human genital cancer. Gynecol. Oncol. 1981;12:S124–S128. - PubMed
-
- Hoots B.E., Palefsky J.M., Pimenta J.M., Smith J.S. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int. J. Cancer J. Int. Du. Cancer. 2009;124:2375–2383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous