Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition
- PMID: 29074488
- PMCID: PMC5866501
- DOI: 10.1152/ajplung.00096.2017
Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition
Abstract
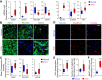
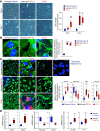
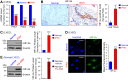
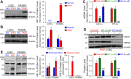
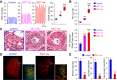
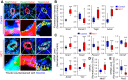
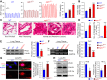
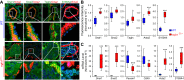
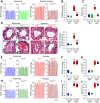
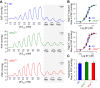
Pulmonary vascular remodeling characterized by concentric wall thickening and intraluminal obliteration is a major contributor to the elevated pulmonary vascular resistance in patients with idiopathic pulmonary arterial hypertension (IPAH). Here we report that increased hypoxia-inducible factor 2α (HIF-2α) in lung vascular endothelial cells (LVECs) under normoxic conditions is involved in the development of pulmonary hypertension (PH) by inducing endothelial-to-mesenchymal transition (EndMT), which subsequently results in vascular remodeling and occlusive lesions. We observed significant EndMT and markedly increased expression of SNAI, an inducer of EndMT, in LVECs from patients with IPAH and animals with experimental PH compared with normal controls. LVECs isolated from IPAH patients had a higher level of HIF-2α than that from normal subjects, whereas HIF-1α was upregulated in pulmonary arterial smooth muscle cells (PASMCs) from IPAH patients. The increased HIF-2α level, due to downregulated prolyl hydroxylase domain protein 2 (PHD2), a prolyl hydroxylase that promotes HIF-2α degradation, was involved in enhanced EndMT and upregulated SNAI1/2 in LVECs from patients with IPAH. Moreover, knockdown of HIF-2α (but not HIF-1α) with siRNA decreases both SNAI1 and SNAI2 expression in IPAH-LVECs. Mice with endothelial cell (EC)-specific knockout (KO) of the PHD2 gene, egln1 (egln1EC-/-), developed severe PH under normoxic conditions, whereas Snai1/2 and EndMT were increased in LVECs of egln1EC-/- mice. EC-specific KO of the HIF-2α gene, hif2a, prevented mice from developing hypoxia-induced PH, whereas EC-specific deletion of the HIF-1α gene, hif1a, or smooth muscle cell (SMC)-specific deletion of hif2a, negligibly affected the development of PH. Also, exposure to hypoxia for 48-72 h increased protein level of HIF-1α in normal human PASMCs and HIF-2α in normal human LVECs. These data indicate that increased HIF-2α in LVECs plays a pathogenic role in the development of severe PH by upregulating SNAI1/2, inducing EndMT, and causing obliterative pulmonary vascular lesions and vascular remodeling.
Keywords: endothelial cell; intimal lesion; prolyl hydroxylase domain-containing protein; pulmonary arterial hypertension.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

