HID-1 controls formation of large dense core vesicles by influencing cargo sorting and trans-Golgi network acidification
- PMID: 29074564
- PMCID: PMC5739301
- DOI: 10.1091/mbc.E17-08-0491
HID-1 controls formation of large dense core vesicles by influencing cargo sorting and trans-Golgi network acidification
Abstract
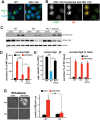
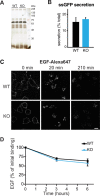
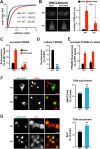
Large dense core vesicles (LDCVs) mediate the regulated release of neuropeptides and peptide hormones. They form at the trans-Golgi network (TGN), where their soluble content aggregates to form a dense core, but the mechanisms controlling biogenesis are still not completely understood. Recent studies have implicated the peripheral membrane protein HID-1 in neuropeptide sorting and insulin secretion. Using CRISPR/Cas9, we generated HID-1 KO rat neuroendocrine cells, and we show that the absence of HID-1 results in specific defects in peptide hormone and monoamine storage and regulated secretion. Loss of HID-1 causes a reduction in the number of LDCVs and affects their morphology and biochemical properties, due to impaired cargo sorting and dense core formation. HID-1 KO cells also exhibit defects in TGN acidification together with mislocalization of the Golgi-enriched vacuolar H+-ATPase subunit isoform a2. We propose that HID-1 influences early steps in LDCV formation by controlling dense core formation at the TGN.
© 2017 Hummer et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




Similar articles
-
Differential sorting behavior for soluble and transmembrane cargoes at the trans-Golgi network in endocrine cells.Mol Biol Cell. 2020 Feb 1;31(3):157-166. doi: 10.1091/mbc.E19-10-0561. Epub 2019 Dec 11. Mol Biol Cell. 2020. PMID: 31825717 Free PMC article.
-
Identification of the functional domain of the dense core vesicle biogenesis factor HID-1.PLoS One. 2023 Sep 26;18(9):e0291977. doi: 10.1371/journal.pone.0291977. eCollection 2023. PLoS One. 2023. PMID: 37751424 Free PMC article.
-
HID-1 is a novel player in the regulation of neuropeptide sorting.Biochem J. 2011 Mar 15;434(3):383-90. doi: 10.1042/BJ20110027. Biochem J. 2011. PMID: 21250940
-
Sorting of neuropeptides and neuropeptide receptors into secretory pathways.Prog Neurobiol. 2010 Feb 9;90(2):276-83. doi: 10.1016/j.pneurobio.2009.10.011. Epub 2009 Oct 22. Prog Neurobiol. 2010. PMID: 19853638 Review.
-
Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model.J Exp Bot. 2016 Aug;67(15):4435-49. doi: 10.1093/jxb/erw222. Epub 2016 Jun 4. J Exp Bot. 2016. PMID: 27262127 Review.
Cited by
-
The priming factor CAPS1 regulates dense-core vesicle acidification by interacting with rabconnectin3β/WDR7 in neuroendocrine cells.J Biol Chem. 2019 Jun 14;294(24):9402-9415. doi: 10.1074/jbc.RA119.007504. Epub 2019 Apr 19. J Biol Chem. 2019. PMID: 31004036 Free PMC article.
-
Control of insulin granule formation and function by the ABC transporters ABCG1 and ABCA1 and by oxysterol binding protein OSBP.Mol Biol Cell. 2018 May 15;29(10):1238-1257. doi: 10.1091/mbc.E17-08-0519. Epub 2018 Mar 22. Mol Biol Cell. 2018. PMID: 29540530 Free PMC article.
-
Developmental and physiological impacts of pathogenic human huntingtin protein in the nervous system.Neurobiol Dis. 2024 Dec;203:106732. doi: 10.1016/j.nbd.2024.106732. Epub 2024 Nov 12. Neurobiol Dis. 2024. PMID: 39542221 Free PMC article.
-
EIPR1 controls dense-core vesicle cargo retention and EARP complex localization in insulin-secreting cells.Mol Biol Cell. 2020 Jan 1;31(1):59-79. doi: 10.1091/mbc.E18-07-0469. Epub 2019 Nov 13. Mol Biol Cell. 2020. PMID: 31721635 Free PMC article.
-
Trafficking of hormones and trophic factors to secretory and extracellular vesicles: a historical perspective and new hypothesis.Extracell Vesicles Circ Nucl Acids. 2023;4(4):568-587. doi: 10.20517/evcna.2023.34. Epub 2023 Nov 9. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 38435713 Free PMC article.
References
-
- Anderson RG, Pathak RK. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985;40:635–643. - PubMed
-
- Argiolas A, Melis MR. Neuropeptides and central control of sexual behaviour from the past to the present: a review. Prog Neurobiol. 2013;108:80–107. - PubMed
-
- Arvan P, Halban PA. Sorting ourselves out: seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

