Phragmoplast microtubule dynamics - a game of zones
- PMID: 29074579
- PMCID: PMC6518215
- DOI: 10.1242/jcs.203331
Phragmoplast microtubule dynamics - a game of zones
Abstract
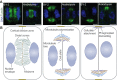
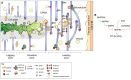
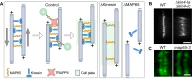
Plant morphogenesis relies on the accurate positioning of the partition (cell plate) between dividing cells during cytokinesis. The cell plate is synthetized by a specialized structure called the phragmoplast, which consists of microtubules, actin filaments, membrane compartments and associated proteins. The phragmoplast forms between daughter nuclei during the transition from anaphase to telophase. As cells are commonly larger than the originally formed phragmoplast, the construction of the cell plate requires phragmoplast expansion. This expansion depends on microtubule polymerization at the phragmoplast forefront (leading zone) and loss at the back (lagging zone). Leading and lagging zones sandwich the 'transition' zone. A population of stable microtubules in the transition zone facilitates transport of building materials to the midzone where the cell plate assembly takes place. Whereas microtubules undergo dynamic instability in all zones, the overall balance appears to be shifted towards depolymerization in the lagging zone. Polymerization of microtubules behind the lagging zone has not been reported to date, suggesting that microtubule loss there is irreversible. In this Review, we discuss: (1) the regulation of microtubule dynamics in the phragmoplast zones during expansion; (2) mechanisms of the midzone establishment and initiation of cell plate biogenesis; and (3) signaling in the phragmoplast.
Keywords: Cell plate; Cytokinesis; Microtubule dynamics; Phragmoplast.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
-
- Asada T., Kuriyama R. and Shibaoka H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110, 179-189. - PubMed
-
- Atmodjo M. A., Sakuragi Y., Zhu X., Burrell A. J., Mohanty S. S., Atwood J. A., Orlando R. III, Scheller H. V. and Mohnen D. (2011). Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan: galacturonosyltransferase complex. Proc. Natl. Acad. Sci. USA 108, 20225-20230. 10.1073/pnas.1112816108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

