Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide
- PMID: 29074595
- PMCID: PMC6410531
- DOI: 10.1182/blood-2017-05-785790
Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide
Abstract
Treatments for high-risk essential thrombocythemia (ET) address thrombocytosis, disease-related symptoms, as well as risks of thrombosis, hemorrhage, transformation to myelofibrosis, and leukemia. Patients resistant/intolerant to hydroxycarbamide (HC) have a poor outlook. MAJIC (ISRCTN61925716) is a randomized phase 2 trial of ruxolitinib (JAK1/2 inhibitor) vs best available therapy (BAT) in ET and polycythemia vera patients resistant or intolerant to HC. Here, findings of MAJIC-ET are reported, where the modified intention-to-treat population included 58 and 52 patients randomized to receive ruxolitinib or BAT, respectively. There was no evidence of improvement in complete response within 1 year reported in 27 (46.6%) patients treated with ruxolitinib vs 23 (44.2%) with BAT (P = .40). At 2 years, rates of thrombosis, hemorrhage, and transformation were not significantly different; however, some disease-related symptoms improved in patients receiving ruxolitinib relative to BAT. Molecular responses were uncommon; there were 2 complete molecular responses (CMR) and 1 partial molecular response in CALR-positive ruxolitinib-treated patients. Transformation to myelofibrosis occurred in 1 CMR patient, presumably because of the emergence of a different clone, raising questions about the relevance of CMR in ET patients. Grade 3 and 4 anemia occurred in 19% and 0% of ruxolitinib vs 0% (both grades) in the BAT arm, and grade 3 and 4 thrombocytopenia in 5.2% and 1.7% of ruxolitinib vs 0% (both grades) of BAT-treated patients. Rates of discontinuation or treatment switching did not differ between the 2 trial arms. The MAJIC-ET trial suggests that ruxolitinib is not superior to current second-line treatments for ET. This trial was registered at www.isrctn.com as #ISRCTN61925716.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.N.H. has participated in advisory boards for Novartis, CTI, Baxaltra, and Celgene; was on speakers bureau for Novartis, CTI, Baxaltra, Shire, Gilead, INCYTE; received honoraria from Novartis, Shire CTI, Gilead, Baxaltra, INCYTE; and received research funding and travel, accommodation, and expenses from Novartis. A.J.M. has participated in advisory boards for Novartis, CTI, and Baxaltra; received honoraria from Novartis, Gilead, Shire, and Baxaltra; and also received research funding and travel, accommodation, and expenses from Novartis. F.C. and J.C. have received travel, accommodation, and expenses from Novartis. S.K. has participated in advisory boards for Novartis; received honoraria from Novartis, Shire, and Gilead; and received travel accommodation and expense from Novartis and Celgene. S. Ali received honoraria from Novartis and participated in advisory boards for Novartis. A.H. has participated in advisory boards for Novartis and was on the speakers bureau from Gilead. N.C.P.C. has participated in advisory boards for Novartis and received honoraria from Novartis and research support from Novartis. R.M. has consulted for Novartis, Ariad, and Galena and received research funding from Incyte, Gilead, CTI, NS Pharma, Celgene, and Promedior. M.F.M. has participated in advisory boards for Novartis and Gilead; received honoraria from Novartis, Shire, and Celgene; and received travel, accommodation, and expenses from Novartis. The remaining authors declare no competing financial interests.
Figures

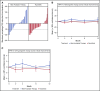
Comment in
-
Ruxolitinib in ET: not all MPN are equal.Blood. 2017 Oct 26;130(17):1873-1874. doi: 10.1182/blood-2017-08-802165. Blood. 2017. PMID: 29074591 No abstract available.
References
-
- Cortelazzo S, Viero P, Finazzi G, D’Emilio A, Rodeghiero F, Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol. 1990;8(3):556-562. - PubMed
-
- Alimam S, Wilkins BS, Harrison CN. How we diagnose and treat essential thrombocythaemia. Br J Haematol. 2015;171(3):306-321. - PubMed
-
- Rumi E, Cazzola M. How I treat essential thrombocythemia. Blood. 2016;128(20):2403-2414. - PubMed
-
- Hernández-Boluda JC, Alvarez-Larran A, Gomez M, et al. . Clinical evaluation of the European LeukaemiaNet criteria for clinicohaematological response and resistance/intolerance to hydroxycarbamide in essential thrombocythaemia. Br J Haematol. 2011;152(1):81-88. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

