LDL Receptor-Related Protein 2 (Megalin) as a Target Antigen in Human Kidney Anti-Brush Border Antibody Disease
- PMID: 29074737
- PMCID: PMC5791069
- DOI: 10.1681/ASN.2017060664
LDL Receptor-Related Protein 2 (Megalin) as a Target Antigen in Human Kidney Anti-Brush Border Antibody Disease
Abstract
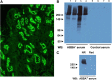
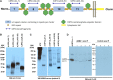
Primary renal tubulointerstitial disease resulting from proximal tubule antigen-specific antibodies and immune complex formation has not been well characterized in humans. We report a cohort of patients with a distinct, underappreciated kidney disease characterized by kidney antibrush border antibodies and renal failure (ABBA disease). We identified ten patients with ABBA disease who had a combination of proximal tubule damage, IgG-positive immune deposits in the tubular basement membrane, and circulating antibodies reactive with normal human kidney proximal tubular brush border. All but one of the patients also had segmental glomerular deposits on renal biopsy specimen. Patients with ABBA disease were elderly and presented with AKI and subnephrotic proteinuria. Serum from all patients but not controls recognized a high molecular weight protein in renal tubular protein extracts that we identified as LDL receptor-related protein 2 (LRP2), also known as megalin, by immunoprecipitation and mass spectrometry. Immunostaining revealed that LRP2 specifically colocalized with IgG in the tubular immune deposits on the ABBA biopsy specimen but not the control specimen analyzed. Finally, ABBA serum samples but not control samples showed reactivity against recombinantly expressed N-terminal LRP2 fragments on Western blots and immunoprecipitated the recombinantly expressed N-terminal region of LRP2. This case series details the clinicopathologic findings of patients with ABBA disease and shows that the antigenic target of these autoantibodies is LRP2. Future studies are needed to determine the disease prevalence, stimulus for ABBA, and optimal treatment.
Keywords: Heymann nephritis; Immunology and pathology; immune complexes; kidney biopsy; kidney tubule; membranous nephropathy.
Copyright © 2018 by the American Society of Nephrology.
Figures



Comment in
-
Tubular disease: Anti-LRP2 nephropathy.Nat Rev Nephrol. 2018 Jan;14(1):3. doi: 10.1038/nrneph.2017.159. Epub 2017 Nov 13. Nat Rev Nephrol. 2018. PMID: 29129926 No abstract available.
References
-
- Sugisaki T, Yoshida T, McCluskey RT, Andres GA, Klassen J: Autoimmune cell-mediated tubulointerstitial nephritis induced in Lewis rats by renal antigens. Clin Immunol Immunopathol 15: 33–43, 1980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

