Methods to Evaluate Cell Growth, Viability, and Response to Treatment in a Tissue Engineered Breast Cancer Model
- PMID: 29074857
- PMCID: PMC5658356
- DOI: 10.1038/s41598-017-14326-8
Methods to Evaluate Cell Growth, Viability, and Response to Treatment in a Tissue Engineered Breast Cancer Model
Abstract
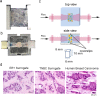
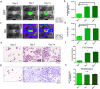
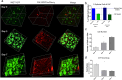
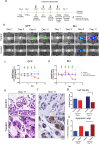
The use of in vitro, engineered surrogates in the field of cancer research is of interest for studies involving mechanisms of growth and metastasis, and response to therapeutic intervention. While biomimetic surrogates better model human disease, their complex composition and dimensionality make them challenging to evaluate in a real-time manner. This feature has hindered the broad implementation of these models, particularly in drug discovery. Herein, several methods and approaches for the real-time, non-invasive analysis of cell growth and response to treatment in tissue-engineered, three-dimensional models of breast cancer are presented. The tissue-engineered surrogates used to demonstrate these methods consist of breast cancer epithelial cells and fibroblasts within a three dimensional volume of extracellular matrix and are continuously perfused with nutrients via a bioreactor system. Growth of the surrogates over time was measured using optical in vivo (IVIS) imaging. Morphologic changes in specific cell populations were evaluated by multi-photon confocal microscopy. Response of the surrogates to treatment with paclitaxel was measured by optical imaging and by analysis of lactate dehydrogenase and caspase-cleaved cytokeratin 18 in the perfused medium. Each method described can be repeatedly performed during culture, allowing for real-time, longitudinal analysis of cell populations within engineered tumor models.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Flow-perfusion bioreactor system for engineered breast cancer surrogates to be used in preclinical testing.J Tissue Eng Regen Med. 2017 Apr;11(4):1242-1250. doi: 10.1002/term.2026. Epub 2015 May 7. J Tissue Eng Regen Med. 2017. PMID: 25950420 Free PMC article.
-
Short term culture of breast cancer tissues to study the activity of the anticancer drug taxol in an intact tumor environment.BMC Cancer. 2006 Apr 7;6:86. doi: 10.1186/1471-2407-6-86. BMC Cancer. 2006. PMID: 16603054 Free PMC article.
-
Preparation and Analysis of In Vitro Three Dimensional Breast Carcinoma Surrogates.J Vis Exp. 2016 May 9;(111):54004. doi: 10.3791/54004. J Vis Exp. 2016. PMID: 27214165 Free PMC article.
-
Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery.Acc Chem Res. 2008 Jan;41(1):139-48. doi: 10.1021/ar7000827. Epub 2007 Jul 27. Acc Chem Res. 2008. PMID: 17655274 Review.
-
Cardiovascular tissue engineering I. Perfusion bioreactors: a review.J Long Term Eff Med Implants. 2006;16(2):111-30. doi: 10.1615/jlongtermeffmedimplants.v16.i2.10. J Long Term Eff Med Implants. 2006. PMID: 16700652 Review.
Cited by
-
Nuclear PDCD4 Expression Defines a Subset of Luminal B-Like Breast Cancers with Good Prognosis.Horm Cancer. 2020 Oct;11(5-6):218-239. doi: 10.1007/s12672-020-00392-4. Epub 2020 Jul 6. Horm Cancer. 2020. PMID: 32632815 Free PMC article.
-
Applicability of drug response metrics for cancer studies using biomaterials.Philos Trans R Soc Lond B Biol Sci. 2019 Aug 19;374(1779):20180226. doi: 10.1098/rstb.2018.0226. Epub 2019 Jul 1. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31431182 Free PMC article.
-
Lung-resident SARS-CoV-2 peptide-specific immune responses in perfused 3D human lung explant models.Front Bioeng Biotechnol. 2025 Jul 8;13:1587080. doi: 10.3389/fbioe.2025.1587080. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40698364 Free PMC article.
-
Spheroid Model of Mammary Tumor Cells: Epithelial-Mesenchymal Transition and Doxorubicin Response.Biology (Basel). 2024 Jun 21;13(7):463. doi: 10.3390/biology13070463. Biology (Basel). 2024. PMID: 39056658 Free PMC article.
-
Engineered models to parse apart the metastatic cascade.NPJ Precis Oncol. 2019 Aug 21;3:20. doi: 10.1038/s41698-019-0092-3. eCollection 2019. NPJ Precis Oncol. 2019. PMID: 31453371 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

