Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity
- PMID: 29074880
- PMCID: PMC5658399
- DOI: 10.1038/s41467-017-01270-4
Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity
Abstract
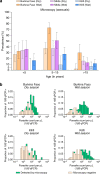
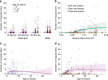
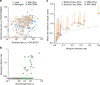
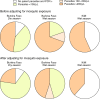
A detailed understanding of the human infectious reservoir is essential for improving malaria transmission-reducing interventions. Here we report a multi-regional assessment of population-wide malaria transmission potential based on 1209 mosquito feeding assays in endemic areas of Burkina Faso and Kenya. Across both sites, we identified 39 infectious individuals. In high endemicity settings, infectious individuals were identifiable by research-grade microscopy (92.6%; 25/27), whilst one of three infectious individuals in the lowest endemicity setting was detected by molecular techniques alone. The percentages of infected mosquitoes in the different surveys ranged from 0.05 (4/7716) to 1.6% (121/7749), and correlate positively with transmission intensity. We also estimated exposure to malaria vectors through genetic matching of blood from 1094 wild-caught bloodfed mosquitoes with that of humans resident in the same houses. Although adults transmitted fewer parasites to mosquitoes than children, they received more mosquito bites, thus balancing their contribution to the infectious reservoir.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

