Differentiation of Oligodendrocyte Precursor Cells from Sox10-Venus Mice to Oligodendrocytes and Astrocytes
- PMID: 29074959
- PMCID: PMC5658394
- DOI: 10.1038/s41598-017-14207-0
Differentiation of Oligodendrocyte Precursor Cells from Sox10-Venus Mice to Oligodendrocytes and Astrocytes
Abstract
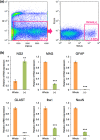
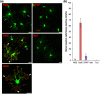
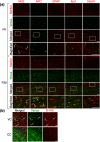
Oligodendrocytes are well known as myelin-forming cells in the central nervous system (CNS). However, detailed mechanisms of oligodendrocyte differentiation and myelination are poorly understood, particularly due to the difficulty of the purification of murine oligodendrocyte precursor cells (OPCs). We have recently established a transgenic mouse line that expresses a fluorescent protein Venus under the promoter of Sox10, whose expression is restricted to OPCs and oligodendrocytes in the CNS. Here, we have characterized Venus-positive cells from the Sox10-Venus mouse brain for analyzing oligodendrocyte differentiation. We first purified Venus-positive cells from the postnatal day 0-2 brain by flow cytometry. Most of the Venus-positive cells expressed NG2, an OPC marker. After induction of differentiation, an increased population of galactocerebroside-positive oligodendrocytes and decrease of OPCs were observed in the Venus-positive culture. Furthermore, a time-lapse analysis showed that Venus-positive oligodendrocytes dynamically changed their morphology with highly branched cell processes during differentiation. In addition, we found that Venus-positive OPCs were able to differentiate to type II astrocytes. In vivo, OPCs and oligodendrocytes express Venus, and some of astrocytes were positive for Venus in the ventral cortex. Taken together, the Sox10-Venus mouse system is useful for analyzing differentiation and multipotency of murine OPCs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

