Trends in GPCR drug discovery: new agents, targets and indications
- PMID: 29075003
- PMCID: PMC6882681
- DOI: 10.1038/nrd.2017.178
Trends in GPCR drug discovery: new agents, targets and indications
Abstract
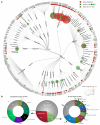
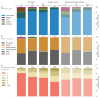
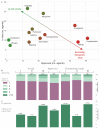
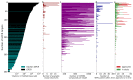
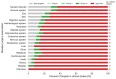
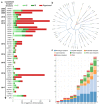
G protein-coupled receptors (GPCRs) are the most intensively studied drug targets, mostly due to their substantial involvement in human pathophysiology and their pharmacological tractability. Here, we report an up-to-date analysis of all GPCR drugs and agents in clinical trials, which reveals current trends across molecule types, drug targets and therapeutic indications, including showing that 475 drugs (~34% of all drugs approved by the US Food and Drug Administration (FDA)) act at 108 unique GPCRs. Approximately 321 agents are currently in clinical trials, of which ~20% target 66 potentially novel GPCR targets without an approved drug, and the number of biological drugs, allosteric modulators and biased agonists has increased. The major disease indications for GPCR modulators show a shift towards diabetes, obesity and Alzheimer disease, although several central nervous system disorders are also highly represented. The 224 (56%) non-olfactory GPCRs that have not yet been explored in clinical trials have broad untapped therapeutic potential, particularly in genetic and immune system disorders. Finally, we provide an interactive online resource to analyse and infer trends in GPCR drug discovery.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Rask-Andersen M, Masuram S, Schiöth HB. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9–26. - PubMed
-
- The IDG Knowledge Management Center. Unexplored opportunities in the druggable human genome. The IDG Knowledge Management Center; 2016. 189205, 189205. [Peer-reviewed poster outlining a major NIH programme to characterise the dark space of major drug target families.]
-
- Kolakowski LF. GCRDb: a G-protein-coupled receptor database. Receptors Channels. 1994;2:1–7. - PubMed
-
- Schiöth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. 2005;142:94–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

