Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities
- PMID: 29075113
- PMCID: PMC5609801
- DOI: 10.2147/IJN.S141709
Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities
Abstract
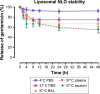
We investigated the efficacy of liposomal gentamicin formulations of different surface charges against Pseudomonas aeruginosa and Klebsiella oxytoca. The liposomal gentamicin formulations were prepared by the dehydration-rehydration method, and their sizes and zeta potential were measured. Gentamicin encapsulation efficiency inside the liposomal formulations was determined by microbiologic assay, and stability of the formulations in biologic fluid was evaluated for a period of 48 h. The minimum inhibitory concentration and the minimum bactericidal concentration were determined, and the in vitro time kill studies of the free form of gentamicin and liposomal gentamicin formulations were performed. The activities of liposomal gentamicin in preventing and reducing biofilm-forming P. aeruginosa and K. oxytoca were compared to those of free antibiotic. The sizes of the liposomal formulations ranged from 625 to 806.6 nm in diameter, with the zeta potential ranging from -0.22 to -31.7 mV. Gentamicin encapsulation efficiency inside the liposomal formulation ranged from 1.8% to 43.6%. The liposomes retained >60% of their gentamicin content during the 48 h time period. The minimum inhibitory concentration of neutral formulation was lower than that of free gentamicin (0.25 versus 1 mg/L for P. aeruginosa and 0.5 versus 1 mg/L for K. oxytoca). The negatively charged formulation exhibited the same bacteriostatic concentration as that of free gentamicin. The minimum bactericidal concentration of neutral liposomes on planktonic bacterial culture was twofold lower than that of free gentamicin, whereas the negatively charged formulations were comparable to free gentamicin. The killing time curve values for the neutral negatively charged formulation against planktonic P. aeruginosa and K. oxytoca were better than those of free gentamicin. Furthermore, liposomal formulations prevent the biofilm-formation ability of these strains better than free gentamicin. In summary, liposomal formulations could be an effective lipid nanoparticle to combat acute infections where planktonic bacteria are predominant.
Keywords: antibacterial activity; biofilm; drug delivery; stability.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures












References
-
- Cohen ML. Changing patterns of infectious disease. Nature. 2000;406(6797):762–767. - PubMed
-
- Yoneyama H, Katsumata R. Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci Biotechnol Biochem. 2006;70(5):1060–1075. - PubMed
-
- Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32(5):675–685. - PubMed
-
- Nathan C. Antibiotics at the crossroads. Nature. 2004;431(7011):899–902. - PubMed
-
- Projan SJ. New (and not so new) antibacterial targets – from where and when will the novel drugs come? Curr Opin Pharmacol. 2002;2(5):513–522. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

