Acinetobacter baumannii Virulence Traits: A Comparative Study of a Novel Sequence Type with Other Italian Endemic International Clones
- PMID: 29075243
- PMCID: PMC5643476
- DOI: 10.3389/fmicb.2017.01977
Acinetobacter baumannii Virulence Traits: A Comparative Study of a Novel Sequence Type with Other Italian Endemic International Clones
Abstract
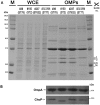
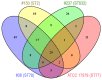
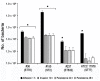
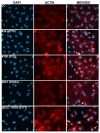
Carbapenem-resistant Acinetobacter baumannii (CRAb) have emerged in recent decades as major causes of nosocomial infections. Resistance is mainly due to overexpression of intrinsic and/or acquired carbapenemases, especially oxacillinases (OXA). In Italy, although the sequence type (ST) 2 and the ST78 are the most frequently detected, we recently reported ST632, a single locus variant of ST2. Therefore, this study was aimed at unraveling common bacterial surface virulence factors involved in pathogenesis and antibiotic resistance in representative CRAb of these ST genotypes. Outer membrane protein (OMP) composition together with motility, biofilm formation, in vitro adherence to, invasion of, and survival within pneumocytes were analyzed. Differently from the carbapenem-susceptible reference strain ATCC 17978, either overexpressed OXA-51 or both OXA-23 and OXA-51 co-purified with OMPs in CRAb. This tight association ensures their maximal concentration on the inner surface of the outer membrane to provide the best protection against carbapenems. These findings led us to propose for the first time a common behavior of OXA enzymes in CRAb. Despite the presence of both OmpA and phosphorylcholine-porinD and the ability of all the strains to adhere to cells, invasion, and survival within pneumocytes was shown only by ST2 and ST78 isolates, sharing the highest number of identified OMPs. Conversely, notwithstanding genetic and OMPs similarities with ST2, ST632 was unable to invade and survive within epithelial cells. Overall, our study shows that different STs share a specific OMP composition, also shaped by overexpressed OXA, that is needed for invasiveness and survival of CRAb.
Keywords: Acinetobacter baumannii; biofilm; host-pathogen interactions; motility; oxacillinases.
Figures


References
-
- Ambrosi C., Aleandri M., Giordano A., Scribano D., Marazzato M., Zagaglia C., et al. (2016). Molecular characterisation of extensively drug-resistant Acinetobacter baumannii: first report of a new sequence type in Italy. J. Glob. Antimicrob. Resist. 7 154–156. 10.1016/j.jgar.2016.10.002 - DOI - PubMed
-
- Choi C. H., Hyun S. H., Lee J. Y., Lee J. S., Lee Y. S., Kim S. A., et al. (2008a). Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell Microbiol. 10 309–319. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

