Vaccine Containing the Three Allelic Variants of the Plasmodium vivax Circumsporozoite Antigen Induces Protection in Mice after Challenge with a Transgenic Rodent Malaria Parasite
- PMID: 29075260
- PMCID: PMC5642139
- DOI: 10.3389/fimmu.2017.01275
Vaccine Containing the Three Allelic Variants of the Plasmodium vivax Circumsporozoite Antigen Induces Protection in Mice after Challenge with a Transgenic Rodent Malaria Parasite
Abstract
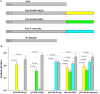
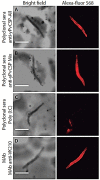
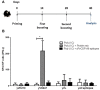
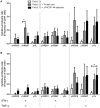
Plasmodium vivax is the most common species that cause malaria outside of the African continent. The development of an efficacious vaccine would contribute greatly to control malaria. Recently, using bacterial and adenoviral recombinant proteins based on the P. vivax circumsporozoite protein (CSP), we demonstrated the possibility of eliciting strong antibody-mediated immune responses to each of the three allelic forms of P. vivax CSP (PvCSP). In the present study, recombinant proteins representing the PvCSP alleles (VK210, VK247, and P. vivax-like), as well as a hybrid polypeptide, named PvCSP-All epitopes, were generated. This hybrid containing the conserved C-terminal of the PvCSP and the three variant repeat domains in tandem were successfully produced in the yeast Pichia pastoris. After purification and biochemical characterization, they were used for the experimental immunization of C57BL/6 mice in a vaccine formulation containing the adjuvant Poly(I:C). Immunization with a recombinant protein expressing all three different allelic forms in fusion elicited high IgG antibody titers reacting with all three different allelic variants of PvCSP. The antibodies targeted both the C-terminal and repeat domains of PvCSP and recognized the native protein on the surface of P. vivax sporozoites. More importantly, mice that received the vaccine formulation were protected after challenge with chimeric Plasmodium berghei sporozoites expressing CSP repeats of P. vivax sporozoites (Pb/PvVK210). Our results suggest that it is possible to elicit protective immunity against one of the most common PvCSP alleles using soluble recombinant proteins expressed by P. pastoris. These recombinant proteins are promising candidates for clinical trials aiming to develop a multiallele vaccine against P. vivax malaria.
Keywords: Plasmodium vivax; circumsporozoite protein; malaria; prime-boost regimens; recombinant vaccine.
Figures



Similar articles
-
Development of a Plasmodium berghei transgenic parasite expressing the full-length Plasmodium vivax circumsporozoite VK247 protein for testing vaccine efficacy in a murine model.Malar J. 2016 Apr 30;15(1):251. doi: 10.1186/s12936-016-1297-3. Malar J. 2016. PMID: 27129682 Free PMC article.
-
Immunogenicity of a prime-boost vaccine containing the circumsporozoite proteins of Plasmodium vivax in rodents.Infect Immun. 2014 Feb;82(2):793-807. doi: 10.1128/IAI.01410-13. Epub 2013 Dec 9. Infect Immun. 2014. PMID: 24478093 Free PMC article.
-
Protective Malaria Vaccine in Mice Based on the Plasmodium vivax Circumsporozoite Protein Fused with the Mumps Nucleocapsid Protein.Vaccines (Basel). 2020 Apr 19;8(2):190. doi: 10.3390/vaccines8020190. Vaccines (Basel). 2020. PMID: 32325874 Free PMC article.
-
A universal vaccine candidate against Plasmodium vivax malaria confers protective immunity against the three PvCSP alleles.Sci Rep. 2021 Sep 9;11(1):17928. doi: 10.1038/s41598-021-96986-1. Sci Rep. 2021. PMID: 34504134 Free PMC article.
-
Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials.Parassitologia. 1999 Sep;41(1-3):397-402. Parassitologia. 1999. PMID: 10697892 Review.
Cited by
-
Genetic polymorphism and natural selection of circumsporozoite protein in Myanmar Plasmodium vivax.Malar J. 2020 Sep 4;19(1):303. doi: 10.1186/s12936-020-03366-7. Malar J. 2020. PMID: 32883283 Free PMC article.
-
Plasmodium vivax vaccine: What is the best way to go?Front Immunol. 2023 Jan 16;13:910236. doi: 10.3389/fimmu.2022.910236. eCollection 2022. Front Immunol. 2023. PMID: 36726991 Free PMC article. Review.
-
Immune System Modulation by the Adjuvants Poly (I:C) and Montanide ISA 720.Front Immunol. 2022 Jun 29;13:910022. doi: 10.3389/fimmu.2022.910022. eCollection 2022. Front Immunol. 2022. PMID: 35844531 Free PMC article.
-
A genetically modified Plasmodium berghei parasite as a surrogate for whole-sporozoite vaccination against P. vivax malaria.NPJ Vaccines. 2022 Dec 16;7(1):163. doi: 10.1038/s41541-022-00585-8. NPJ Vaccines. 2022. PMID: 36526627 Free PMC article.
-
Optimization of an in vivo model to study immunity to Plasmodium falciparum pre-erythrocytic stages.Malar J. 2019 Dec 18;18(1):426. doi: 10.1186/s12936-019-3055-9. Malar J. 2019. PMID: 31849326 Free PMC article.
References
-
- WHO. World Malaria Report 2015. Geneva: WHO Library; (2016).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

