Aluminum, a Friend or Foe of Higher Plants in Acid Soils
- PMID: 29075280
- PMCID: PMC5643487
- DOI: 10.3389/fpls.2017.01767
Aluminum, a Friend or Foe of Higher Plants in Acid Soils
Abstract
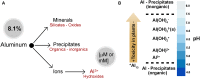
Aluminum (Al) is the most abundant metal in the earth's crust, but its availability depends on soil pH. Despite this abundance, Al is not considered an essential element and so far no experimental evidence has been put forward for a biological role. In plants and other organisms, Al can have a beneficial or toxic effect, depending on factors such as, metal concentration, the chemical form of Al, growth conditions and plant species. Here we review recent advances in the study of Al in plants at physiological, biochemical and molecular levels, focusing mainly on the beneficial effect of Al in plants (stimulation of root growth, increased nutrient uptake, the increase in enzyme activity, and others). In addition, we discuss the possible mechanisms involved in improving the growth of plants cultivated in soils with acid pH, as well as mechanisms of tolerance to the toxic effect of Al.
Keywords: acid soils; aluminum; aluminum toxicity; beneficial effect of aluminum; mechanisms of tolerance; metal; plant growth stimulation.
Figures

References
-
- Andrivon D. (1995). Inhibition by aluminum of mycelia growth and of sporangial production and germination in Phytophthora infestans. Eur. J. Plant Pathol. 101 527–533. 10.1007/BF01874477 - DOI
-
- Anitha S., Rao K. S. J. (2002). The complexity of aluminum-DNA interactions: relevance to Alzheimer’s and other neurological diseases. Struct. Bond. 104 79–97. 10.1007/3-540-45425-X_3 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

