Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer
- PMID: 29075294
- PMCID: PMC5623803
- DOI: 10.1155/2017/5035371
Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer
Abstract
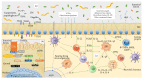
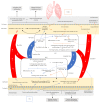
The microbiota includes different microorganisms consisting of bacteria, fungi, viruses, and protozoa distributed over many human body surfaces including the skin, vagina, gut, and airways, with the highest density found in the intestine. The gut microbiota strongly influences our metabolic, endocrine, and immune systems, as well as both the peripheral and central nervous systems. Recently, a dialogue between the gut and lung microbiota has been discovered, suggesting that changes in one compartment could impact the other compartment, whether in relation to microbial composition or function. Further, this bidirectional axis is evidenced in an, either beneficial or malignant, altered immune response in one compartment following changes in the other compartment. Stimulation of the immune system arises from the microbial cells themselves, but also from their metabolites. It can be either direct or mediated by stimulated immune cells in one site impacting the other site. Additionally, this interaction may lead to immunological boost, assisting the innate immune system in its antitumour response. Thus, this review offers an insight into the composition of these sites, the gut and the lung, their role in shaping the immune system, and, finally, their role in the response to lung cancer.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

