Cardiac Progenitor Cells and the Interplay with Their Microenvironment
- PMID: 29075298
- PMCID: PMC5623801
- DOI: 10.1155/2017/7471582
Cardiac Progenitor Cells and the Interplay with Their Microenvironment
Abstract
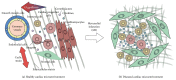
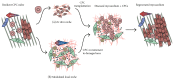
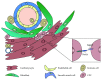
The microenvironment plays a crucial role in the behavior of stem and progenitor cells. In the heart, cardiac progenitor cells (CPCs) reside in specific niches, characterized by key components that are altered in response to a myocardial infarction. To date, there is a lack of knowledge on these niches and on the CPC interplay with the niche components. Insight into these complex interactions and into the influence of microenvironmental factors on CPCs can be used to promote the regenerative potential of these cells. In this review, we discuss cardiac resident progenitor cells and their regenerative potential and provide an overview of the interactions of CPCs with the key elements of their niche. We focus on the interaction between CPCs and supporting cells, extracellular matrix, mechanical stimuli, and soluble factors. Finally, we describe novel approaches to modulate the CPC niche that can represent the next step in recreating an optimal CPC microenvironment and thereby improve their regeneration capacity.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

