Rhodosporidium toruloides: a new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts
- PMID: 29075325
- PMCID: PMC5651578
- DOI: 10.1186/s13068-017-0927-5
Rhodosporidium toruloides: a new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts
Abstract
Background: Economical conversion of lignocellulosic biomass into biofuels and bioproducts is central to the establishment of a robust bioeconomy. This requires a conversion host that is able to both efficiently assimilate the major lignocellulose-derived carbon sources and divert their metabolites toward specific bioproducts.
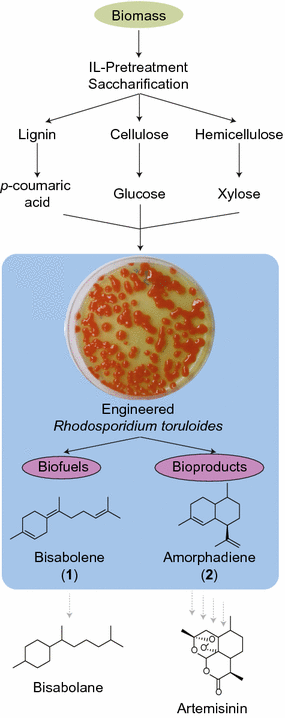
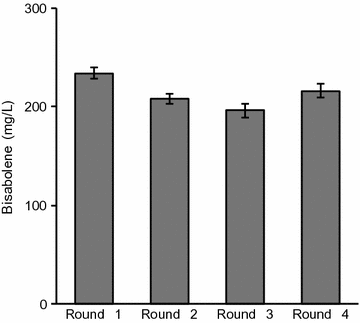
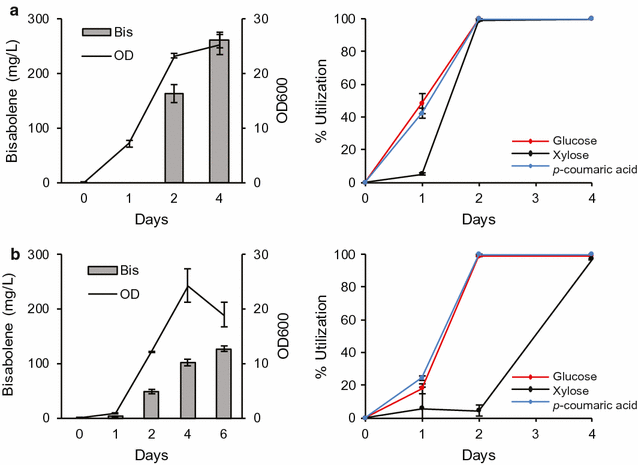
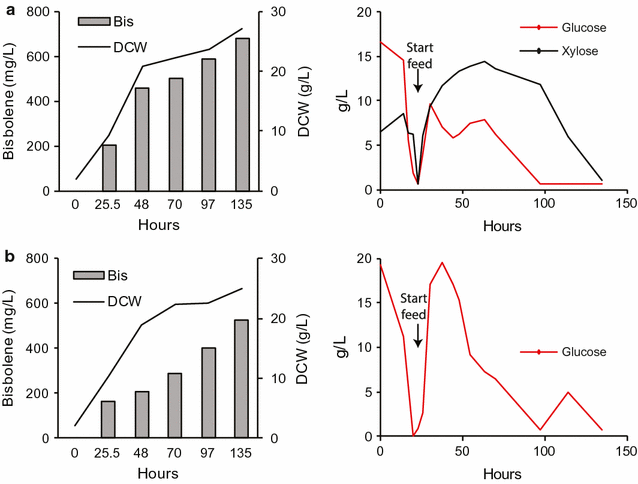
Results: In this study, the carotenogenic yeast Rhodosporidium toruloides was examined for its ability to convert lignocellulose into two non-native sesquiterpenes with biofuel (bisabolene) and pharmaceutical (amorphadiene) applications. We found that R. toruloides can efficiently convert a mixture of glucose and xylose from hydrolyzed lignocellulose into these bioproducts, and unlike many conventional production hosts, its growth and productivity were enhanced in lignocellulosic hydrolysates relative to purified substrates. This organism was demonstrated to have superior growth in corn stover hydrolysates prepared by two different pretreatment methods, one using a novel biocompatible ionic liquid (IL) choline α-ketoglutarate, which produced 261 mg/L of bisabolene at bench scale, and the other using an alkaline pretreatment, which produced 680 mg/L of bisabolene in a high-gravity fed-batch bioreactor. Interestingly, R. toruloides was also observed to assimilate p-coumaric acid liberated from acylated grass lignin in the IL hydrolysate, a finding we verified with purified substrates. R. toruloides was also able to consume several additional compounds with aromatic motifs similar to lignin monomers, suggesting that this organism may have the metabolic potential to convert depolymerized lignin streams alongside lignocellulosic sugars.
Conclusions: This study highlights the natural compatibility of R. toruloides with bioprocess conditions relevant to lignocellulosic biorefineries and demonstrates its ability to produce non-native terpenes.
Keywords: Amorphadiene; Bisabolene; Heterologous expression; Multiple carbon source utilization; Plant biomass-derived hydrolysate; Rhodosporidium toruloides; Terpenes.
Figures




References
-
- Clark JH, Budarin V, Deswarte FEI, Hardy JJE, Kerton FM, Hunt AJ, Luque R, Macquarrie DJ, Milkowski K, Rodriguez A, Samuel O, Tavener SJ, White RJ, Wilson AJ. Green chemistry and the biorefinery: a partnership for a sustainable future. Green Chem. 2006;8:853. doi: 10.1039/b604483m. - DOI
-
- Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wymann CE. Lignin valorization: improving lignin processing in the biorefinery. Science. 2014;344:1246843. doi: 10.1126/science.1246843. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

