Rethinking cancer nanotheranostics
- PMID: 29075517
- PMCID: PMC5654564
- DOI: 10.1038/natrevmats.2017.24
Rethinking cancer nanotheranostics
Abstract
Advances in nanoparticle synthesis and engineering have produced nanoscale agents affording both therapeutic and diagnostic functions that are often referred to by the portmanteau 'nanotheranostics'. The field is associated with many applications in the clinic, especially in cancer management. These include patient stratification, drug-release monitoring, imaging-guided focal therapy and post-treatment response monitoring. Recent advances in nanotheranostics have expanded this notion and enabled the characterization of individual tumours, the prediction of nanoparticle-tumour interactions, and the creation of tailor-designed nanomedicines for individualized treatment. Some of these applications require breaking the dogma that a nanotheranostic must combine both therapeutic and diagnostic agents within a single, physical entity; instead, it can be a general approach in which diagnosis and therapy are interwoven to solve clinical issues and improve treatment outcomes. In this Review, we describe the evolution and state of the art of cancer nanotheranostics, with an emphasis on clinical impact and translation.
Conflict of interest statement
Competing interests statement The authors declare no competing interests.
Figures


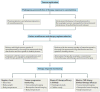
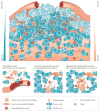

References
-
- Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. This perspective stimulates an interesting discussion on the efficiency of nanoparticle delivery to tumours.
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
