Elimination of Mycoplasma Contamination from Infected Human Hepatocyte C3A Cells by Intraperitoneal Injection in BALB/c Mice
- PMID: 29075618
- PMCID: PMC5643414
- DOI: 10.3389/fcimb.2017.00440
Elimination of Mycoplasma Contamination from Infected Human Hepatocyte C3A Cells by Intraperitoneal Injection in BALB/c Mice
Abstract
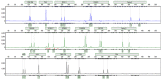
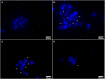
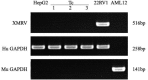
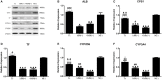
Background/Aims: The use of antibiotics to eliminate Mycoplasma contamination has some serious limitations. Mycoplasma contamination can be eliminated by intraperitoneal injection of BALB/c mice with contaminated cells combined with screening monoclonal cells. However, in vivo passage in mice after injection with contaminated cells requires a long duration (20-54 days). Furthermore, it is important to monitor for cross-contamination of mouse and human cells, xenotropic murine leukemia virus-related virus (XMRV) infection, and altered cell function after the in vivo treatment. The present study aimed to validate a reliable and simplified method to eliminate mycoplasma contamination from human hepatocytes. BALB/c mice were injected with paraffin oil prior to injection with cells, in order to shorten duration of intraperitoneal passage. Cross-contamination of mouse and human cells, XMRV infection and cell function-related genes and proteins were also evaluated. Methods: PCR and DNA sequencing were used to confirm Mycoplasma hyorhinis (M. hyorhinis) contamination in human hepatocyte C3A cells. Five BALB/c mice were intraperitoneally injected with 0.5 ml paraffin oil 1 week before injection of the cells. The mice were then intraperitoneally injected with C3A hepatocytes (5.0 × 106/ml) contaminated with M. hyorhinis (6.2 ± 2.2 × 108 CFU/ml). Ascites were collected for monoclonal cell screening on the 14th day after injection of contaminated cells. Elimination of mycoplasma from cells was determined by PCR and Transmission Electron Microscopy (TEM). Human-mouse cell and XMRV contamination were also detected by PCR. Quantitative reverse transcription PCR and western blotting were used to compare the expression of genes and proteins among treated cells, non-treated infected cells, and uninfected cells. Results: Fourteen days after injection with cells, 4 of the 5 mice had ascites. Hepatocyte colonies extracted from the ascites of four mice were all mycoplasma-free. There was no cell cross-contamination or XMRV infection in treated cell cultures. Elimination of Mycoplasma resulted in partial or complete recovery in the expression of ALB, TF, and CYP3A4 genes as well as proteins. Proliferation of the treated cells was not significantly affected by this management. Conclusion: The method of elimination of Mycoplasma contamination in this study was validated and reproducible. Success was achieved in four of five cases examined. Compared to the previous studies, the duration of intraperitoneal passage in this study was significantly shorter.
Keywords: Mycoplasma; cell cross-contamination; elimination; intraperitoneal inoculation; monoclonal cells.
Figures




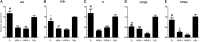

Similar articles
-
Elimination of mycoplasmas from mouse myeloma cells by intraperitoneal passage in mice and by antibiotic treatment.Hybridoma. 1989 Apr;8(2):249-57. doi: 10.1089/hyb.1989.8.249. Hybridoma. 1989. PMID: 2714817
-
Decontamination of mycoplasma-contaminated Orientia tsutsugamushi strains by repeating passages through cell cultures with antibiotics.BMC Microbiol. 2013 Feb 8;13:32. doi: 10.1186/1471-2180-13-32. BMC Microbiol. 2013. PMID: 23394970 Free PMC article.
-
Effective treatment of mycoplasma contamination in cell lines with enrofloxacin (Baytril).Leukemia. 1994 Aug;8(8):1424-34. Leukemia. 1994. PMID: 7520103
-
N-Terminal Polypeptide of Annexin A2 Decreases Infection of Mycoplasma hyorhinis to Gastric Cancer Cells.PLoS One. 2016 Jan 26;11(1):e0147776. doi: 10.1371/journal.pone.0147776. eCollection 2016. PLoS One. 2016. PMID: 26812398 Free PMC article.
-
Detection of Mycoplasma Contamination in Nanoparticle Formulations: Version 3.2020 Sep. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method STE-3. 2020 Sep. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method STE-3. PMID: 39012980 Free Books & Documents. Review.
Cited by
-
iTRAQ-based Comparative Serum Proteomic Analysis of Prostate Cancer Patients with or without Bone Metastasis.J Cancer. 2019 Jul 10;10(18):4165-4177. doi: 10.7150/jca.33497. eCollection 2019. J Cancer. 2019. PMID: 31413735 Free PMC article.
-
Effects and Eradication of Mycoplasma Contamination on Patient-derived Colorectal Cancer Organoid Cultures.Cancer Res Commun. 2023 Sep 27;3(9):1952-1958. doi: 10.1158/2767-9764.CRC-23-0109. Cancer Res Commun. 2023. PMID: 37772998 Free PMC article.
-
Anti-Mycoplasma Activity of Daptomycin and Its Use for Mycoplasma Elimination in Cell Cultures of Rickettsiae.Antibiotics (Basel). 2019 Aug 21;8(3):123. doi: 10.3390/antibiotics8030123. Antibiotics (Basel). 2019. PMID: 31438510 Free PMC article.
-
Preparation of mouse anti-human rotavirus VP7 monoclonal antibody and its protective effect on rotavirus infection.Exp Ther Med. 2019 Aug;18(2):1384-1390. doi: 10.3892/etm.2019.7708. Epub 2019 Jun 25. Exp Ther Med. 2019. PMID: 31384336 Free PMC article.
-
Programmable Synthetic Protein Circuits for the Identification and Suppression of Hepatocellular Carcinoma.Mol Ther Oncolytics. 2020 Mar 30;17:70-82. doi: 10.1016/j.omto.2020.03.008. eCollection 2020 Jun 26. Mol Ther Oncolytics. 2020. PMID: 32322664 Free PMC article.
References
-
- Beutler E., Gelbart T., Lee P., Trevino R., Fernandez M. A., Fairbanks V. F. (2000). Molecular characterization of a case of atransferrinemia. Blood 96, 4071–4074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

