Citalopram Ameliorates Impairments in Spatial Memory and Synaptic Plasticity in Female 3xTgAD Mice
- PMID: 29075638
- PMCID: PMC5624171
- DOI: 10.1155/2017/1238687
Citalopram Ameliorates Impairments in Spatial Memory and Synaptic Plasticity in Female 3xTgAD Mice
Abstract
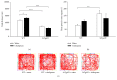
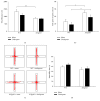
Alzheimer's disease (AD) is the primary cause of dementia. There is no effective treatment. Amyloid-β peptide (Aβ) plays an important role in the pathogenesis and thus strategies suppressing Aβ production and accumulation seem promising. Citalopram is an antidepressant drug and can decrease Aβ production and amyloid plaques in transgenic mice of AD and humans. Whether citalopram can ameliorate memory deficit was not known yet. We tested the effects of citalopram on behavioral performance and synaptic plasticity in female 3xTgAD mice, a well-characterized model of AD. Mice were treated with citalopram or water from 5 months of age for 3 months. Citalopram treatment at approximately 10 mg/kg/day significantly improved spatial memory in the Morris water maze (MWM) test, while not affecting anxiety-like and depression-like behavior in 3xTgAD mice. Further, hippocampal long-term potentiation (LTP) impairment in 3xTgAD mice was reversed by citalopram treatment. Citalopram treatment also significantly decreased the levels of insoluble Aβ40 in hippocampal and cortical tissues in 3xTgAD mice, accompanied with a reduced amyloid precursor protein (APP). Together, citalopram treatment may be a promising strategy for AD and further clinical trials should be conducted to verify the effect of citalopram on cognition in patients with AD or mild cognitive impairment.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

