Predicting in vivo effect levels for repeat-dose systemic toxicity using chemical, biological, kinetic and study covariates
- PMID: 29075892
- PMCID: PMC5818596
- DOI: 10.1007/s00204-017-2067-x
Predicting in vivo effect levels for repeat-dose systemic toxicity using chemical, biological, kinetic and study covariates
Abstract
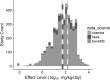
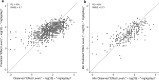
In an effort to address a major challenge in chemical safety assessment, alternative approaches for characterizing systemic effect levels, a predictive model was developed. Systemic effect levels were curated from ToxRefDB, HESS-DB and COSMOS-DB from numerous study types totaling 4379 in vivo studies for 1247 chemicals. Observed systemic effects in mammalian models are a complex function of chemical dynamics, kinetics, and inter- and intra-individual variability. To address this complex problem, systemic effect levels were modeled at the study-level by leveraging study covariates (e.g., study type, strain, administration route) in addition to multiple descriptor sets, including chemical (ToxPrint, PaDEL, and Physchem), biological (ToxCast), and kinetic descriptors. Using random forest modeling with cross-validation and external validation procedures, study-level covariates alone accounted for approximately 15% of the variance reducing the root mean squared error (RMSE) from 0.96 log10 to 0.85 log10 mg/kg/day, providing a baseline performance metric (lower expectation of model performance). A consensus model developed using a combination of study-level covariates, chemical, biological, and kinetic descriptors explained a total of 43% of the variance with an RMSE of 0.69 log10 mg/kg/day. A benchmark model (upper expectation of model performance) was also developed with an RMSE of 0.5 log10 mg/kg/day by incorporating study-level covariates and the mean effect level per chemical. To achieve a representative chemical-level prediction, the minimum study-level predicted and observed effect level per chemical were compared reducing the RMSE from 1.0 to 0.73 log10 mg/kg/day, equivalent to 87% of predictions falling within an order-of-magnitude of the observed value. Although biological descriptors did not improve model performance, the final model was enriched for biological descriptors that indicated xenobiotic metabolism gene expression, oxidative stress, and cytotoxicity, demonstrating the importance of accounting for kinetics and non-specific bioactivity in predicting systemic effect levels. Herein, we generated an externally predictive model of systemic effect levels for use as a safety assessment tool and have generated forward predictions for over 30,000 chemicals.
Keywords: Effect levels; Predictive toxicity; Systemic effects.
Figures



References
-
- Commission E (2013) Ban on animal testing. http://ec.europa.eu/consumers/consumers_safety/cosmetics/ban_on_animal_t.... Accessed 11-12-2014
-
- ECHA (2006) REACH—registration, evaluation, authorisation and restriction of chemicals. http://ec.europa.eu/enterprise/sectors/chemicals/reach/index_en.htm. Accessed October 30, 2014
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

