A systematic autopsy survey of human infant bridging veins
- PMID: 29075919
- PMCID: PMC5807502
- DOI: 10.1007/s00414-017-1714-3
A systematic autopsy survey of human infant bridging veins
Abstract
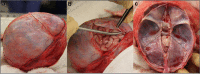
In the first years of life, subdural haemorrhage (SDH) within the cranial cavity can occur through accidental and non-accidental mechanisms as well as from birth-related injury. This type of bleeding is the most common finding in victims of abusive head trauma (AHT). Historically, the most frequent cause of SDHs in infancy is suggested to be traumatic damage to bridging veins traversing from the brain to the dural membrane. However, several alternative hypotheses have been suggested for the cause and origin of subdural bleeding. It has also been suggested by some that bridging veins are too large to rupture through the forces associated with AHT. To date, there have been no systematic anatomical studies on infant bridging veins. During 43 neonatal, infant and young child post-mortem examinations, we have mapped the locations and numbers of bridging veins onto a 3D model of the surface of a representative infant brain. We have also recorded the in situ diameter of 79 bridging veins from two neonatal, one infant and two young children at post-mortem examination. Large numbers of veins, both distant from and directly entering the dural venous sinuses, were discovered travelling between the brain and dural membrane, with the mean number of veins per brain being 54.1 and the largest number recorded as 94. The mean diameter of the bridging veins was 0.93 mm, with measurements ranging from 0.05 to 3.07 mm. These data demonstrate that some veins are extremely small and subjectively, and they appear to be delicate. Characterisation of infant bridging veins will contribute to the current understanding of potential vascular sources of subdural bleeding and could also be used to further develop computational models of infant head injury.
Keywords: Abuse; Bridging vein; Child; Head injury; Post-mortem; Subdural haemorrhage.
Conflict of interest statement
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Retrospective ethical approval was given for the use of anonymised archived autopsy images for the purpose of 3D anatomical mapping of bridging veins. For cases involving the use of digital photography for vein measuring purposes, and MRI scanning, appropriate parental consent was prospectively obtained.
Conflict of interest
RDGM regularly provides expert opinion to various Courts in relation to paediatric head injury and receives payment for doing so. Otherwise, the authors declare that they have no conflict of interest.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

