The Use of Plant-Derived Ribosome Inactivating Proteins in Immunotoxin Development: Past, Present and Future Generations
- PMID: 29076988
- PMCID: PMC5705959
- DOI: 10.3390/toxins9110344
The Use of Plant-Derived Ribosome Inactivating Proteins in Immunotoxin Development: Past, Present and Future Generations
Abstract
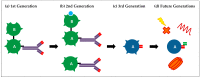
Ribosome inactivating proteins (RIPs) form a class of toxins that was identified over a century ago. They continue to fascinate scientists and the public due to their very high activity and long-term stability which might find useful applications in the therapeutic killing of unwanted cells but can also be used in acts of terror. We will focus our review on the canonical plant-derived RIPs which display ribosomal RNA N-glycosidase activity and irreversibly inhibit protein synthesis by cleaving the 28S ribosomal RNA of the large 60S subunit of eukaryotic ribosomes. We will place particular emphasis on therapeutic applications and the generation of immunotoxins by coupling antibodies to RIPs in an attempt to target specific cells. Several generations of immunotoxins have been developed and we will review their optimisation as well as their use and limitations in pre-clinical and clinical trials. Finally, we endeavour to provide a perspective on potential future developments for the therapeutic use of immunotoxins.
Keywords: immunotoxins; ribosome inactivating proteins; therapeutic applications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

