Bacterial Expression of Human Butyrylcholinesterase as a Tool for Nerve Agent Bioscavengers Development
- PMID: 29077024
- PMCID: PMC6150354
- DOI: 10.3390/molecules22111828
Bacterial Expression of Human Butyrylcholinesterase as a Tool for Nerve Agent Bioscavengers Development
Abstract
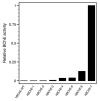
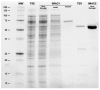
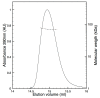
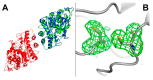
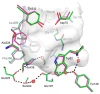
Human butyrylcholinesterase is a performant stoichiometric bioscavenger of organophosphorous nerve agents. It is either isolated from outdated plasma or functionally expressed in eukaryotic systems. Here, we report the production of active human butyrylcholinesterase in a prokaryotic system after optimization of the primary sequence through the Protein Repair One Stop Shop process, a structure- and sequence-based algorithm for soluble bacterial expression of difficult eukaryotic proteins. The mutant enzyme was purified to homogeneity. Its kinetic parameters with substrate are similar to the endogenous human butyrylcholinesterase or recombinants produced in eukaryotic systems. The isolated protein was prone to crystallize and its 2.5-Å X-ray structure revealed an active site gorge region identical to that of previously solved structures. The advantages of this alternate expression system, particularly for the generation of butyrylcholinesterase variants with nerve agent hydrolysis activity, are discussed.
Keywords: 3D structure; PROSS; SEC-MALS; butyrylcholinesterase; differential scanning fluorimetry; prokaryotic expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
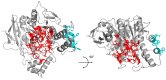

References
-
- Lenz D.E., Maxwell D.M., Koplovitz I., Clark C.R., Capacio B.R., Cerasoli D.M., Federko J.M., Luo C., Saxena A., Doctor B.P., et al. Protection against soman or VX poisoning by human butyrylcholinesterase in guinea pigs and cynomolgus monkeys. Chem. Biol. Interact. 2005;157–158:205–210. doi: 10.1016/j.cbi.2005.10.025. - DOI - PubMed
-
- Carmona G.N., Jufer R.A., Goldberg S.R., Gorelick D.A., Greig N.H., Yu Q.S., Cone E.J., Schindler C.W. Butyrylcholinesterase accelerates cocaine metabolism: In vitro and in vivo effects in nonhuman primates and humans. Drug Metab. Dispos. 2000;28:367–371. - PubMed
-
- Kosak U., Knez D., Coquelle N., Brus B., Pislar A., Nachon F., Brazzolotto X., Kos J., Colletier J.P., Gobec S. N-propargylpiperidines with naphthalene-2-carboxamide or naphthalene-2-sulfonamide moieties: Potential multifunctional anti-alzheimer’s agents. Bioorg. Med. Chem. 2017;25:633–645. doi: 10.1016/j.bmc.2016.11.032. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

