Micro free flow electrophoresis
- PMID: 29077103
- PMCID: PMC5819367
- DOI: 10.1039/c7lc01105a
Micro free flow electrophoresis
Abstract
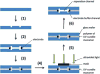
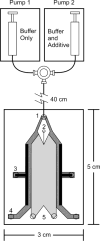
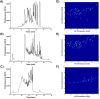
Micro free-flow electrophoresis (μFFE) is a continuous separation technique in which analytes are streamed through a perpendicularly applied electric field in a planar separation channel. Analyte streams are deflected laterally based on their electrophoretic mobilities as they flow through the separation channel. A number of μFFE separation modes have been demonstrated, including free zone (FZ), micellar electrokinetic chromatography (MEKC), isoelectric focusing (IEF) and isotachophoresis (ITP). Approximately 60 articles have been published since the first μFFE device was fabricated in 1994. We anticipate that recent advances in device design, detection, and fabrication, will allow μFFE to be applied to a much wider range of applications. Applications particularly well suited for μFFE analysis include continuous, real time monitoring and microscale purifications.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures








References
-
- Barrollier J, Watzke E, Gibian H. Z Naturforsch B. 1958;13:754–756. - PubMed
-
- Hannig K. Fresen Z Anal Chem. 1961;181:244–254.
-
- Hannig K. J Chromatogr. 1978;159:183–191. - PubMed
-
- Křivánková L, Boček P. Electrophoresis. 1998;19:1064–1074. - PubMed
-
- Islinger M, Eckerskorn C, Völkl A. Electrophoresis. 2010;31:1754–1763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

