Contribution of the β-glucosidase BglC to the onset of the pathogenic lifestyle of Streptomyces scabies
- PMID: 29077242
- PMCID: PMC6638027
- DOI: 10.1111/mpp.12631
Contribution of the β-glucosidase BglC to the onset of the pathogenic lifestyle of Streptomyces scabies
Abstract
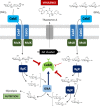
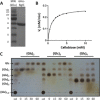
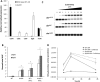
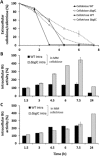
Common scab disease on root and tuber plants is caused by Streptomyces scabies and related species which use the cellulose synthase inhibitor thaxtomin A as the main phytotoxin. Thaxtomin production is primarily triggered by the import of cello-oligosaccharides. Once inside the cell, the fate of the cello-oligosaccharides is dichotomized: (i) the fuelling of glycolysis with glucose for the saprophytic lifestyle through the action of β-glucosidase(s) (BGs); and (ii) elicitation of the pathogenic lifestyle by the inhibition of CebR-mediated transcriptional repression of thaxtomin biosynthetic genes. Here, we investigated the role of scab57721, encoding a putative BG (BglC), in the onset of the pathogenicity of S. scabies. Enzymatic assays showed that BglC was able to release glucose from cellobiose, cellotriose and all other cello-oligosaccharides tested. Its inactivation resulted in a phenotype opposite to that expected, as reduced production of thaxtomin was monitored when the mutant was cultivated on medium containing cello-oligosaccharides as unique carbon source. This unexpected phenotype could be attributed to the highly increased activity of alternative intracellular BGs, probably as a compensation for bglC inactivation, which then prevented cellobiose and cellotriose accumulation to reduce the activity of CebR. In contrast, when the bglC null mutant was cultivated on medium devoid of cello-oligosaccharides, it instead constitutively produced thaxtomin. This observed hypervirulent phenotype does not fit with the proposed model of the cello-oligosaccharide-mediated induction of thaxtomin production, and suggests that the role of BglC in the route to the pathogenic lifestyle of S. scabies is more complex than currently presented.
Keywords: CebR; cello-oligosaccharides; common scab disease; thaxtomin; β-glucosidase.
© 2017 BSPP AND JOHN WILEY & SONS LTD.
Figures



References
-
- Bignell, D.R.D. , Huguet‐Tapia, J. , Joshi, M.V. , Pettis, G.S. and Loria, R. (2010) What does it take to be a plant pathogen: genomic insights from Streptomyces species. Antonie Van Leeuwenhoek, 98, 179–194. - PubMed
-
- Francis, I.M. , Jourdan, S. , Fanara, S. , Loria, R. and Rigali, S. (2015) The cellobiose sensor CebR is the gatekeeper of Streptomyces scabies pathogenicity. mBio, 6, e02018–e02014. http://mbio.asm.org/content/6/2/e02018-14.full - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

