Phenotypic Characterization of Corneal Endothelial Dystrophy in German Shorthaired and Wirehaired Pointers Using In Vivo Advanced Corneal Imaging and Histopathology
- PMID: 29077583
- PMCID: PMC5799001
- DOI: 10.1097/ICO.0000000000001431
Phenotypic Characterization of Corneal Endothelial Dystrophy in German Shorthaired and Wirehaired Pointers Using In Vivo Advanced Corneal Imaging and Histopathology
Abstract
Purpose: To evaluate corneal morphology using ultrasonic pachymetry (USP), Fourier-domain optical coherence tomography (FD-OCT), and in vivo confocal microscopy (IVCM) in 2 related canine breeds-German shorthaired pointers (GSHPs) and German wirehaired pointers (GWHPs)-with and without corneal endothelial dystrophy (CED). This condition is characterized by premature endothelial cell degeneration leading to concomitant corneal edema and is similar to Fuchs endothelial corneal dystrophy.
Methods: Corneas of 10 CED-affected (4 GSHP and 6 GWHP) and 19 unaffected, age-matched (15 GSHP and 4 GWHP) dogs were examined using USP, FD-OCT, and IVCM. A 2-sample t test or Mann-Whitney rank-sum test was used to statistically compare parameters between both groups. Data are presented as mean ± SD or median (range).
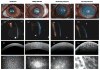
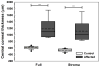
Results: Central corneal thickness determined using USP was significantly greater in CED-affected than in unaffected dogs at 1179 (953-1959) and 646 (497-737) μm, respectively (P < 0.001). Central epithelial thickness was found to be significantly decreased in CED-affected versus unaffected dogs at 47 ± 7.1 and 55 ± 7.1 μm, respectively (P = 0.011), using FD-OCT. With IVCM, corneal endothelial density was significantly less (P < 0.001) in 5 dogs with CED versus 19 unaffected controls at 499 ± 315 versus 1805 ± 298 cells/mm, respectively. CED-affected dogs exhibited endothelial pleomorphism and polymegethism, whereas CED-unaffected dogs had regular hexagonal arrangement of cells.
Conclusions: GSHPs and GWHPs with CED exhibit marked differences in corneal morphology when compared with age-matched control dogs. These 2 CED-affected breeds represent spontaneous, large animal models for human Fuchs endothelial corneal dystrophy.
Conflict of interest statement
Conflict of interest: None
Figures





References
-
- Chi HH, Teng CC, Katzin HM. Histopathology of primary endothelial-epithelial dystrophy of the cornea. Am J Ophthalmol. 1958;45:518–535. - PubMed
-
- Gwin RM, Polack FM, Warren JK, et al. Primary canine corneal endothelial cell dystrophy: Specular microscopic evaluation, diagnosis and therapy. Journal of the American Animal Hospital Association. 1982;18:471–479.
-
- Martin CL, Dice PF. Corneal endothelial dystrophy in the dog. J Am Anim Hosp Assoc. 1982;18:327–336.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

