PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease
- PMID: 29077793
- PMCID: PMC5841141
- DOI: 10.1093/brain/awx258
PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease
Abstract
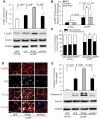
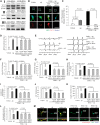
Mitochondrial dysfunction and synaptic damage are early pathological features of the Alzheimer's disease-affected brain. Memory impairment in Alzheimer's disease is a manifestation of brain pathologies such as accumulation of amyloid-β peptide and mitochondrial damage. The underlying pathogenic mechanisms and effective disease-modifying therapies for Alzheimer's disease remain elusive. Here, we demonstrate for the first time that decreased PTEN-induced putative kinase 1 (PINK1) expression is associated with Alzheimer's disease pathology. Restoring neuronal PINK1 function strikingly reduces amyloid-β levels, amyloid-associated pathology, oxidative stress, as well as mitochondrial and synaptic dysfunction. In contrast, PINK1-deficient mAPP mice augmented cerebral amyloid-β accumulation, mitochondrial abnormalities, impairments in learning and memory, as well as synaptic plasticity at an earlier age than mAPP mice. Notably, gene therapy-mediated PINK1 overexpression promotes the clearance of damaged mitochondria by augmenting autophagy signalling via activation of autophagy receptors (OPTN and NDP52), thereby alleviating amyloid-β-induced loss of synapses and cognitive decline in Alzheimer's disease mice. Loss of PINK1 activity or blockade of PINK1-mediated signalling (OPTN or NDP52) fails to reverse amyloid-β-induced detrimental effects. Our findings highlight a novel mechanism by which PINK1-dependent signalling promotes the rescue of amyloid pathology and amyloid-β-mediated mitochondrial and synaptic dysfunctions in a manner requiring activation of autophagy receptor OPTN or NDP52. Thus, activation of PINK1 may represent a new therapeutic avenue for combating Alzheimer's disease.
Keywords: Aβ; PINK1; autophagy; mitochondrial dysfunction; synaptic injury.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures






References
-
- Abramowski D, Rabe S, Upadhaya AR, Reichwald J, Danner S, Staab D, et al.Transgenic expression of intraneuronal Abeta42 but not Abeta40 leads to cellular Abeta lesions, degeneration, and functional impairment without typical Alzheimer's disease pathology. J Neurosci 2012; 32: 1273–83. - PMC - PubMed
-
- Akizuki M, Yamashita H, Uemura K, Maruyama H, Kawakami H, Ito H, et al.Optineurin suppression causes neuronal cell death via NF-kappaB pathway. J Neurochem 2013; 126: 699–704. - PubMed
-
- Barrachina M, Maes T, Buesa C, Ferrer I. Lysosome-associated membrane protein 1 (LAMP-1) in Alzheimer's disease. Neuropathol Appl Neurobiol 2006; 32: 505–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

