Psychological treatments for people with epilepsy
- PMID: 29078005
- PMCID: PMC6485515
- DOI: 10.1002/14651858.CD012081.pub2
Psychological treatments for people with epilepsy
Abstract
Background: Given the significant impact epilepsy can have on the health-related quality of life (HRQoL) of individuals with epilepsy and their families, there is great clinical interest in evidence-based psychological treatments, aimed at enhancing psychological well-being in people with epilepsy. A review of the current evidence was needed to assess the effects of psychological treatments for people with epilepsy on HRQoL outcomes, in order to inform future therapeutic recommendations and research designs.
Objectives: To assess the effects of psychological treatments for people with epilepsy on HRQoL outcomes.
Search methods: We searched the following databases on 20 September 2016, without language restrictions: Cochrane Epilepsy Group Specialized Register, CENTRAL, MEDLINE PsycINFO, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP). We screened the references from included studies and relevant reviews, and contacted researchers in the field for unpublished studies.
Selection criteria: We considered randomized controlled trials (RCTs) and quasi-RCTs for this review. HRQoL was the main outcome measure. For the operational definition of 'psychological treatments', we included a broad range of treatments that used psychological or behavioral techniques designed to improve HRQoL, seizure frequency and severity, and psychiatric comorbidities for adults and children with epilepsy, compared to treatment as usual (TAU) or an active control group.
Data collection and analysis: We used standard methodological procedures expected by the Cochrane Collaboration.
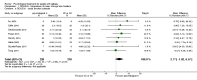
Main results: We included 24 completed RCTs, with a total of 2439 participants. Eleven studies investigated psychological interventions, such as cognitive, behavioral, and mindfulness-based interventions. The remaining studies were classified as educational interventions (N = 7), self-management interventions (N = 3), adherence interventions (N = 1), and mixed interventions (N = 2). Two studies investigated interventions for children and adolescents, and five studies investigated interventions for adolescents and adults. Based on satisfactory clinical and methodological homogeneity, we pooled data from six adult studies, two studies on adolescents and adults, and one on adolescents and young adults (468 participants) for HRQoL, measured with the Quality of Life in Epilepsy-31 (QOLIE-31). We found significant mean changes for the QOLIE-31 total score and six subscales (emotional well-being, energy and fatigue, overall QoL, seizure worry, medication effects, and cognitive functioning). The mean changes of the QOLIE-31 total score (mean improvement of 5.68 points (95% CI 3.11 to 8.24; P < 0.0001), and three subscales, emotional well-being (mean improvement of 7.03 points (95% CI 2.51 to 11.54; P = 0.002); energy and Fatigue (mean improvement of 6.90 points (95% CI 3.49 to 10.31; P < 0.0001); and overall QoL (mean improvement of 6.47 points (95% CI 2.68 to 10.25; P = 0.0008) exceeded the threshold of minimally important change (MIC), indicating a clinically meaningful post-intervention improvement of QoL. We downgraded the quality of the evidence provided by the meta-analysis because of serious risk of bias in some of the included studies. Consequentially, these results provided evidence of moderate quality that psychological treatments for adults with epilepsy may enhance overall QoL in people with epilepsy.
Authors' conclusions: Implications for practice: Psychological interventions and self-management interventions improved QoL, and emotional well-being, and reduced fatigue in adults and adolescents with epilepsy. Adjunctive use of psychological treatments for adults and adolescents with epilepsy may provide additional benefits to QoL in those who incorporate patient-centered management.
Implications for research: Authors should strictly adhere to the CONSORT guidelines to improve the quality of reporting on their interventions. A thorough description of the intervention protocol is necessary to ensure reproducibility.When researching psychological treatments for people with epilepsy, the use of Quality of Life in Epilepsy Inventories (QOLIE-31, QOLIE-31-P, and QOLIE-89) would increase comparability. There is a critical gap in pediatric RCTs for psychological treatments, particularly those that use an epilepsy-specific measure of HRQoL.Finally, in order to increase the overall quality of study designs, adequate randomization with allocation concealment and blinded outcome assessment should be pursued when conducting RCTs. As attrition is often high in research that requires active participant participation, an intention-to-treat analysis should be carried out.
Conflict of interest statement
RM: Dr. Michaelis has a research position at the Gemeinschaftskrankenhaus Herdecke/CURAM/University Witten/Herdecke that allows her to follow her scientific interests, which included working on the Cochrane review. The research position is funded by a grant from the MAHLE foundation and the ICURAM. The grant includes the reimbursement of travel expenses that are related to the content of her scientific work. Prior to the MAHLE foundation (July 2014), the grant money was provided by the Christophorus foundation.
VT: Dr. Tang received a travel stipend from the ILAE to attend the 31st International Epilepsy Congress in Istanbul Turkey (September 2015), during which the Task Force had a one‐day meeting related to the study.
JW: Dr. Wagner received a travel stipend from the ILAE to attend the 31st International Epilepsy Congress in Istanbul Turkey (September 2015), during which the Task Force had a one‐day meeting related to the study.
AM: Dr. Modi received research funding from NIH and was a consultant to Fish and Richardson regarding adherence to medications in adults with multiple sclerosis. She received a travel stipend from the ILAE to attend the 31st International Epilepsy Congress in Istanbul Turkey (September 2015), during which the Task Force had a one‐day meeting related to the study.
WCL: Dr. LaFrance works on this Cochrane project that addressed evidence‐based interventions for epilepsy reviewed by the ILAE committee. He received travel stipend from the ILAE to attend the 31st International Epilepsy Congress in Istanbul Turkey (September 2015), during which the Task Force had a one‐day meeting related to the study. Dr. LaFrance receives author royalties for the seizure treatment book, Taking Control of Your Seizures: Workbook, Oxford University Press, 2015. He studies evidence‐based non‐pharmacological interventions for patients with seizures that are ethics committee approved and peer reviewed to address any potential bias.
LG: Dr. Goldstein has received honoraria for speaking, and educational activities not funded by industry; she receives royalties from the publication of Clinical Neuropsychology (Wiley, 2004, 2013) and The Clinical Psychologist’s Handbook of Epilepsy Cull 1997. This work represents independent research part‐funded by the NIHR Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust and the Institute of Psychiatry, Psychology and Neuroscience, King’s College London. The views expressed are those of the author, and not necessarily those of the NHS, the NIHR or the Department of Health. The views expressed are those of the authors, and not necessarily those of the NHS, the NIHR, or the Department of Health.
TL: None known.
MR: Dr Reuber is responsible for developing and supervising a team of psychotherapists working in a clinical neurology department and provides treatment to patients with epilepsy. Therefore, he has an interest in demonstrating the effectiveness of psychotherapy. However, this potential bias is outweighed by his interest in the development of evidence‐based treatments, encouraging him to assess the existing evidence as objectively and impartially as possible.
Figures
















Update of
References
References to studies included in this review
-
- Beretta S, Beghi E, Messina P, Gerardi F, Pescini F, Licata A, et al. Comprehensive educational plan for patients with epilepsy and comorbidity (EDU‐COM): a pragmatic randomised trial. Journal of Neurology, Neurosurgery, and Psychiatry 2014;85(8):889‐94. [DOI: 10.1136/jnnp-2013-306553; ISSN: 1468‐330X 0022‐3050; PUBMED: 24403284] - DOI - PubMed
-
- Caller T, Ferguson R, Roth R, Secore K, Alexandre F, Zhao W, et al. Self‐management (HOBSCOTCH) improves cognition and quality of life in epilepsy: a randomised controlled trial. Neurology 2016;86(16 Supplement):Abstract no: S22.005.
- Caller TA, Ferguson RJ, Roth RM, Secore KL, Alexandre FP, Zhao W, et al. A cognitive behavioral intervention (HOBSCOTCH) improves quality of life and attention in epilepsy. Epilepsy & Behavior 2016;57(Part A):111‐7. [DOI: 10.1016/j.yebeh.2016.01.024] - DOI - PubMed
-
- Chaytor N, Ciechanowski P, Miller JW, Fraser R, Russo J, Unutzer J, et al. Long‐term outcomes from the PEARLS randomised trial for the treatment of depression in patients with epilepsy. Epilepsy & Behavior 2011;20(3):545‐9. [DOI: 10.1016/j.yebeh.2011.01.017; ISSN: 1525‐5069; PUBMED: 21333607] - DOI - PubMed
- Ciechanowski P, Chaytor N, Miller J, Fraser R, Russo J, Unutzer J, et al. PEARLS depression treatment for individuals with epilepsy: a randomised controlled trial. Epilepsy & Behavior 2010;19(3):225‐31. [DOI: 10.1016/j.yebeh.2010.06.003; ISSN: 1525‐5069; PUBMED: 20609631] - DOI - PubMed
-
- DiIorio C, Bamps Y, Walker E. Results of a randomised controlled trial evaluating WebEase, an online self‐management program. Epilepsy Currents 2011;11(Suppl 1):Abstract no: 2.358.
- DiIorio C, Bamps Y, Walker ER, Escoffery C. Results of a research study evaluating WebEase, an online epilepsy self‐management program. Epilepsy & Behavior 2011;22(3):469‐74. [DOI: 10.1016/j.yebeh.2011.07.030; ISSN: 1525‐5069; PUBMED: 21889413] - DOI - PubMed
References to studies excluded from this review
-
- Dahl J, Melin L, Brorson LO, Schollin J. Effects of a broad‐spectrum behavior modification treatment program on children with refractory epileptic seizures. Epilepsia 1985;26(4):303‐9. [PUBMED: 4006888] - PubMed
-
- Dahl J, Brorson LO, Melin L. Effects of a broad‐spectrum behavioral medicine treatment program on children with refractory epileptic seizures: an 8‐year follow‐up. Epilepsia 1992;33(1):98‐102. [PUBMED: 1733765] - PubMed
-
- Davis GR, Armstrong HE Jr, Donovan DM, Temkin NR. Cognitive‐behavioral treatment of depressed affect among epileptics: preliminary findings. Journal of Clinical Psychology 1984;40(4):930‐5. [PUBMED: 6434595] - PubMed
References to ongoing studies
-
- Kralj‐Hans I, Goldstein LH, Noble AJ, Landau S, Magill N, McCrone P, et al. Self‐Management education for adults with poorly controlled epILEpsy (SMILE (UK)): a randomised controlled trial protocol. BMC Neurology 2014;14:69. [DOI: 10.1186/1471-2377-14-69; PUBMED: 24694207] - DOI - PMC - PubMed
- Ridsdale L. Self‐management education for adults with poorly controlled epilepsy: the SMILE trial. European Journal of Neurology 2015;22(Suppl 1):118, Abstract no: L101. [DOI: 10.1111/ene.12806] - DOI
- Ridsdale L, Kralj‐Hans I, Noble A, Landau S, McCrone P, Morgan M, et al. Self‐management education for epilepsy: an RCT protocol. Journal of Neurology, Neurosurgery and Psychiatry2014; Vol. 85, issue 10:A20‐A21, Abstract no: 076. [DOI: 10.1136/jnnp-2014-309236.76] - DOI
Additional references
-
- Alper K, Schwartz KA, Kolts RL, Khan A. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biological Psychiatry 2007;62(4):345‐54. - PubMed
-
- Baldwin SA, Imel Z. Therapist effects. In: Lambert MJ editor(s). Handbook of Psychotherapy and Behavioral Change. New York: Wiley, 2013:258‐97.
-
- Benavente‐Aguilar I, Morales‐Blanquez C, Rubio EA, Rey JM. Quality of life of adolescents suffering from epilepsy living in the community. Journal of Paediatric Child Health 2004;40(3):110‐3. - PubMed
-
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT Group. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine 2008;148(4):W60‐6. [PUBMED: 18283201] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

