Bioengineering a non-genotoxic vector for genetic modification of mesenchymal stem cells
- PMID: 29078136
- PMCID: PMC5671363
- DOI: 10.1016/j.biomaterials.2017.10.028
Bioengineering a non-genotoxic vector for genetic modification of mesenchymal stem cells
Abstract
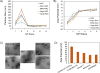
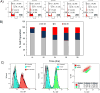
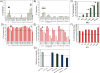
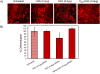
Vectors used for stem cell transfection must be non-genotoxic, in addition to possessing high efficiency, because they could potentially transform normal stem cells into cancer-initiating cells. The objective of this research was to bioengineer an efficient vector that can be used for genetic modification of stem cells without any negative somatic or genetic impact. Two types of multifunctional vectors, namely targeted and non-targeted were genetically engineered and purified from E. coli. The targeted vectors were designed to enter stem cells via overexpressed receptors. The non-targeted vectors were equipped with MPG and Pep1 cell penetrating peptides. A series of commercial synthetic non-viral vectors and an adenoviral vector were used as controls. All vectors were evaluated for their efficiency and impact on metabolic activity, cell membrane integrity, chromosomal aberrations (micronuclei formation), gene dysregulation, and differentiation ability of stem cells. The results of this study showed that the bioengineered vector utilizing VEGFR-1 receptors for cellular entry could transfect mesenchymal stem cells with high efficiency without inducing genotoxicity, negative impact on gene function, or ability to differentiate. Overall, the vectors that utilized receptors as ports for cellular entry (viral and non-viral) showed considerably better somato- and genosafety profiles in comparison to those that entered through electrostatic interaction with cellular membrane. The genetically engineered vector in this study demonstrated that it can be safely and efficiently used to genetically modify stem cells with potential applications in tissue engineering and cancer therapy.
Keywords: Cell transfection; Genotoxicity; Nanoparticles; Non-viral; Stem cells; Vector engineering.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None
Figures




References
-
- Uhl M, Weiler M, Wick W, Jacobs AH, Weller M, Herrlinger U. Migratory neural stem cells for improved thymidine kinase-based gene therapy of malignant gliomas. Biochem Biophys Res Commun. 2005;328(1):125–9. - PubMed
-
- Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67(13):6304–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

