STAT3-RXR-Nrf2 activates systemic redox and energy homeostasis upon steep decline in pO2 gradient
- PMID: 29078168
- PMCID: PMC5680518
- DOI: 10.1016/j.redox.2017.10.013
STAT3-RXR-Nrf2 activates systemic redox and energy homeostasis upon steep decline in pO2 gradient
Abstract
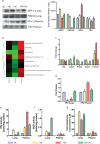
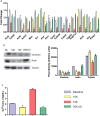
Hypobaric hypoxia elicits several patho-physiological manifestations, some of which are known to be lethal. Among various molecular mechanisms proposed so far, perturbation in redox state due to imbalance between radical generation and antioxidant defence is promising. These molecular events are also related to hypoxic status of cancer cells and therefore its understanding has extended clinical advantage beyond high altitude hypoxia. In present study, however, the focus was to understand and propose a model for rapid acclimatization of high altitude visitors to enhance their performance based on molecular changes. We considered using simulated hypobaric hypoxia at some established thresholds of high altitude stratification based on known physiological effects. Previous studies have focused on the temporal aspect while overlooking the effects of varying pO2 levels during exposure to hypobaric hypoxia. The pO2 levels, indicative of altitude, are crucial to redox homeostasis and can be the limiting factor during acclimatization to hypobaric hypoxia. In this study we present the effects of acute (24h) exposure to high (3049m; pO2: 71kPa), very high (4573m; pO2: 59kPa) and extreme altitude (7620m; pO2: 40kPa) zones on lung and plasma using semi-quantitative redox specific transcripts and quantitative proteo-bioinformatics workflow in conjunction with redox stress assays. It was observed that direct exposure to extreme altitude caused 100% mortality, which turned into high survival rate after pre-exposure to 59kPa, for which molecular explanation were also found. The pO2 of 59kPa (very high altitude zone) elicits systemic energy and redox homeostatic processes by modulating the STAT3-RXR-Nrf2 trio. Finally we posit the various processes downstream of STAT3-RXR-Nrf2 and the plasma proteins that can be used to ascertain the redox status of an individual.
Keywords: Cytoskeleton; Energy homeostasis; Hypoxia; Network biology; Nrf2; RXR; Redox homeostasis; STAT3; pO(2) gradient.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures






References
-
- Blumen I.J., Rinnert K.J. Altitude physiology and the stresses of flight. Air Med. J. 1995;14:87. - PubMed
-
- Nguyen M.H., Jafri M.S. Mitochondrial calcium signaling and energy metabolism. Ann. N.Y. Acad. Sci. 2005;1047:127. - PubMed
-
- Catrina S.-B., Okamoto K., Pereira T., Brismar K., Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1α protein stability and function. Diabetes. 2004;53:3226. - PubMed
-
- Yamada N. Genetic variation in the hypoxia-inducible factor-1α gene is associated with type 2 diabetes in Japanese. J. Clin. Endocrinol. Metab. 2005;90:5841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

