Control of transcriptional activity by design of charge patterning in the intrinsically disordered RAM region of the Notch receptor
- PMID: 29078291
- PMCID: PMC5676888
- DOI: 10.1073/pnas.1706083114
Control of transcriptional activity by design of charge patterning in the intrinsically disordered RAM region of the Notch receptor
Abstract
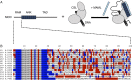
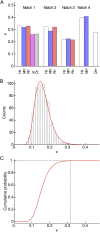
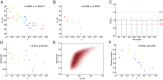
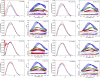
Intrinsically disordered regions (IDRs) play important roles in proteins that regulate gene expression. A prominent example is the intracellular domain of the Notch receptor (NICD), which regulates the transcription of Notch-responsive genes. The NICD sequence includes an intrinsically disordered RAM region and a conserved ankyrin (ANK) domain. The 111-residue RAM region mediates bivalent interactions of NICD with the transcription factor CSL. Although the sequence of RAM is poorly conserved, the linear patterning of oppositely charged residues shows minimal variation. The conformational properties of polyampholytic IDRs are governed as much by linear charge patterning as by overall charge content. Here, we used sequence design to assess how changing the charge patterning within RAM affects its conformational properties, the affinity of NICD to CSL, and Notch transcriptional activity. Increased segregation of oppositely charged residues leads to linear decreases in the global dimensions of RAM and decreases the affinity of a construct including a C-terminal ANK domain (RAMANK) for CSL. Increasing charge segregation from WT RAM sharply decreases transcriptional activation for all permutants. Activation also decreases for some, but not all, permutants with low charge segregation, although there is considerable variation. Our results suggest that the RAM linker is more than a passive tether, contributing local and/or long-range sequence features that modulate interactions within NICD and with downstream components of the Notch pathway. We propose that sequence features within IDRs have evolved to ensure an optimal balance of sequence-encoded conformational properties, interaction strengths, and cellular activities.
Keywords: Notch signaling; ankyrin repeats; intrinsically disordered proteins; sequence design; transcriptional activation.
Published under the PNAS license.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

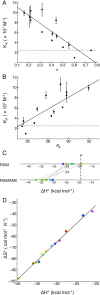



References
-
- Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269:2–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

