Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets
- PMID: 29078402
- PMCID: PMC5703324
- DOI: 10.1073/pnas.1714896114
Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets
Abstract
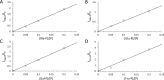
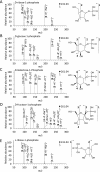
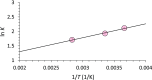
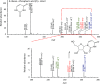
Phosphorylation is an essential chemical reaction for life. This reaction generates fundamental cell components, including building blocks for RNA and DNA, phospholipids for cell walls, and adenosine triphosphate (ATP) for energy storage. However, phosphorylation reactions are thermodynamically unfavorable in solution. Consequently, a long-standing question in prebiotic chemistry is how abiotic phosphorylation occurs in biological compounds. We find that the phosphorylation of various sugars to form sugar-1-phosphates can proceed spontaneously in aqueous microdroplets containing a simple mixture of sugars and phosphoric acid. The yield for d-ribose-1-phosphate reached over 6% at room temperature, giving a ΔG value of -1.1 kcal/mol, much lower than the +5.4 kcal/mol for the reaction in bulk solution. The temperature dependence of the product yield for the phosphorylation in microdroplets revealed a negative enthalpy change (ΔH = -0.9 kcal/mol) and a negligible change of entropy (ΔS = 0.0007 kcal/mol·K). Thus, the spontaneous phosphorylation reaction in microdroplets occurred by overcoming the entropic hurdle of the reaction encountered in bulk solution. Moreover, uridine, a pyrimidine ribonucleoside, is generated in aqueous microdroplets containing d-ribose, phosphoric acid, and uracil, which suggests the possibility that microdroplets could serve as a prebiotic synthetic pathway for ribonucleosides.
Keywords: microdroplet chemistry; origin of life; prebiotic chemistry; sugar phosphorylation; uracil ribosylation.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Comment in
-
Prebiotic phosphorylation enabled by microdroplets.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):12359-12361. doi: 10.1073/pnas.1717373114. Epub 2017 Nov 7. Proc Natl Acad Sci U S A. 2017. PMID: 29114047 Free PMC article. No abstract available.
References
-
- Gull M. Prebiotic phosphorylation reactions on the early earth. Challenges. 2014;5:193–212.
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
-
- Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. - PubMed
-
- Tozzi MG, Camici M, Mascia L, Sgarrella F, Ipata PL. Pentose phosphates in nucleoside interconversion and catabolism. FEBS J. 2006;273:1089–1101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

