Genome-wide analysis of wheat calcium ATPases and potential role of selected ACAs and ECAs in calcium stress
- PMID: 29078753
- PMCID: PMC5658947
- DOI: 10.1186/s12870-017-1112-5
Genome-wide analysis of wheat calcium ATPases and potential role of selected ACAs and ECAs in calcium stress
Abstract
Background: P2- type calcium ATPases (ACAs-auto inhibited calcium ATPases and ECAs-endoplasmic reticulum calcium ATPases) belong to the P- type ATPase family of active membrane transporters and are significantly involved in maintaining accurate levels of Ca2+, Mn2+ and Zn2+ in the cytosol as well as playing a very important role in stress signaling, stomatal opening and closing and pollen tube growth. Here we report the identification and possible role of some of these ATPases from wheat.
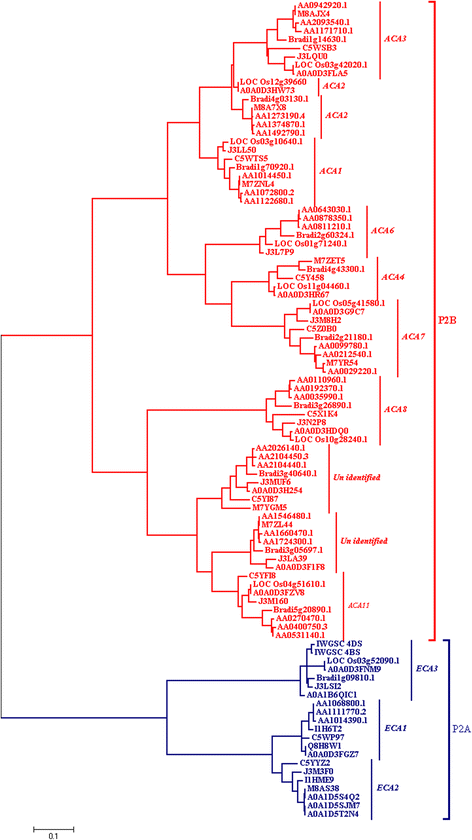
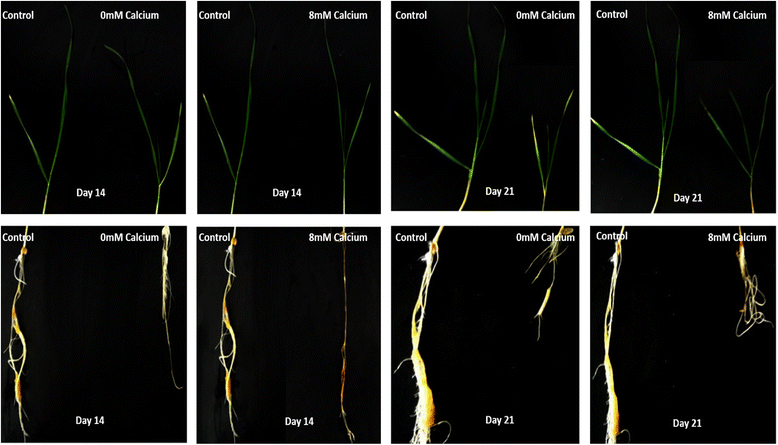
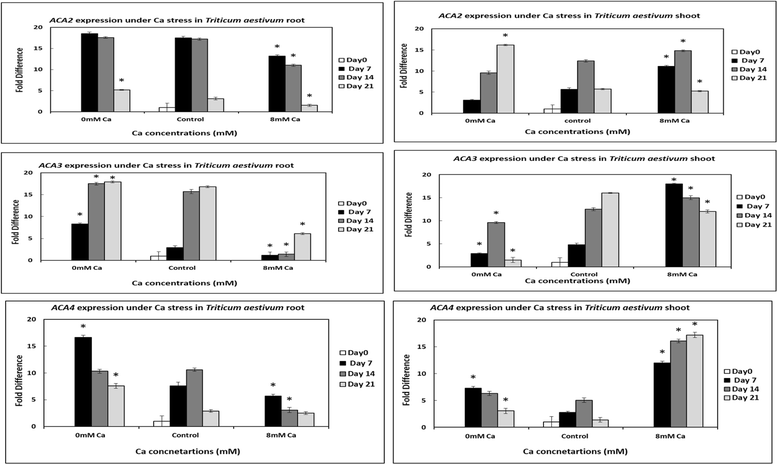
Results: In this study, ACA and ECA sequences of six species (belonging to Poaceae) were retrieved from different databases and a phylogenetic tree was constructed. A high degree of evolutionary relatedness was observed among P2 sequences characterized in this study. Members of the respective groups from different plant species were observed to fall under the same clade. This pattern highlights the common ancestry of P2- type calcium ATPases. Furthermore, qRT-PCR was used to analyse the expression of selected ACAs and ECAs from Triticum aestivum (wheat) under calcium toxicity and calcium deficiency. The data indicated that expression of ECAs is enhanced under calcium stress, suggesting possible roles of these ATPases in calcium homeostasis in wheat. Similarly, the expression of ACAs was significantly different in plants grown under calcium stress as compared to plants grown under control conditions. This gives clues to the role of ACAs in signal transduction during calcium stress in wheat.
Conclusion: Here we concluded that wheat genome consists of nine P2B and three P2A -type calcium ATPases. Moreover, gene loss events in wheat ancestors lead to the loss of a particular homoeolog of a gene in wheat. To elaborate the role of these wheat ATPases, qRT-PCR was performed. The results indicated that when plants are exposed to calcium stress, both P2A and P2B gene expression get enhanced. This further gives clues about the possible role of these ATPases in wheat in calcium management. These findings can be useful in future for genetic manipulations as well as in wheat genome annotation process.
Keywords: ACAs; Calcium; ECAs; P2- type; qRT-PCR.
Conflict of interest statement
Ethics approval and consent to participate
Dr. Alvina Gul Kazi from “Wheat Wide Crosses” lab at National Agriculture and Research Council (NARC) Pakistan identified and provided Wheat seeds (var. Sehar-06). Sehar-06 is a widely cultivated wheat variety in Pakistan.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

