Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies
- PMID: 29078802
- PMCID: PMC5658952
- DOI: 10.1186/s13287-017-0688-x
Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies
Abstract
Background: Degenerative diseases are a major public health concern for the aging population and mesenchymal stem cells (MSCs) have great potential for treating many of these diseases. However, the quantity and quality of MSCs declines with aging, limiting the potential efficacy of autologous MSCs for treating the elderly population.
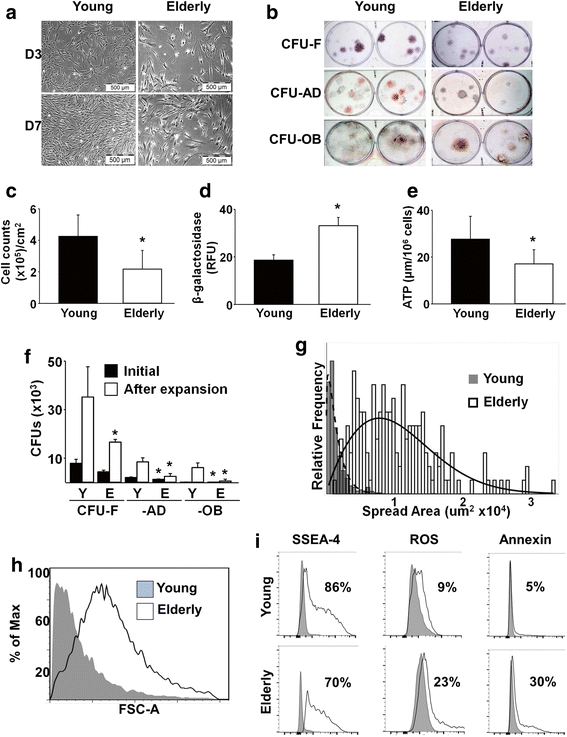
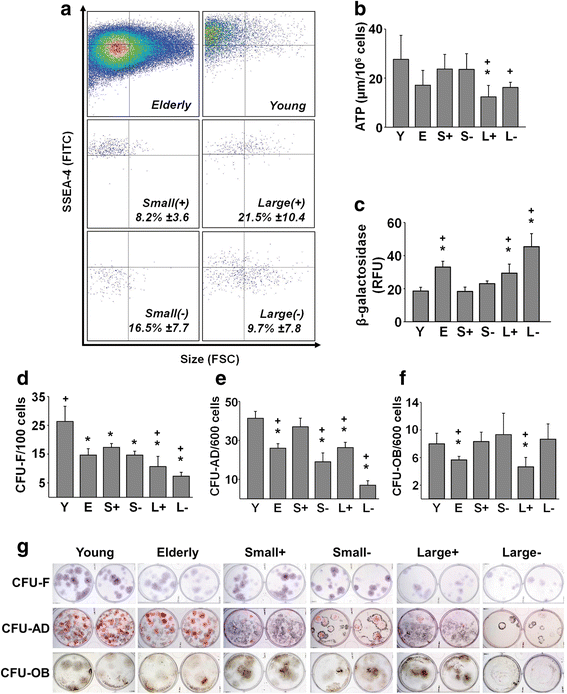
Methods: Human bone marrow (BM)-derived MSCs from young and elderly donors were obtained and characterized using standard cell surface marker criteria (CD73, CD90, CD105) as recommended by the International Society for Cellular Therapy (ISCT). The elderly MSC population was isolated into four subpopulations based on size and stage-specific embryonic antigen-4 (SSEA-4) expression using fluorescence-activated cell sorting (FACS), and subpopulations were compared to the unfractionated young and elderly MSCs using assays that evaluate MSC proliferation, quality, morphology, intracellular reactive oxygen species, β-galactosidase expression, and adenosine triphosphate (ATP) content.
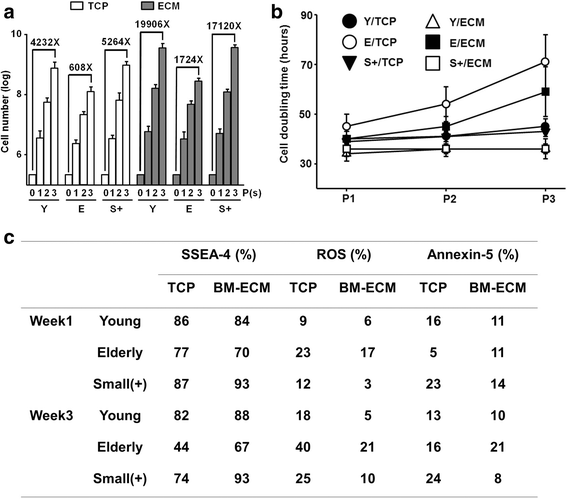
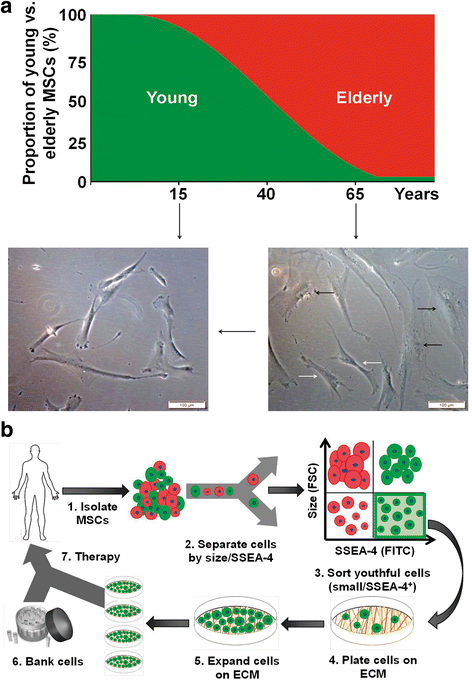
Results: The ISCT-recommended cell surface markers failed to detect any differences between young and elderly MSCs. Here, we report that elderly MSCs were larger in size and displayed substantially higher concentrations of intracellular reactive oxygen species and β-galactosidase expression and lower amounts of ATP and SSEA-4 expression. Based on these findings, cell size and SSEA-4 expression were used to separate the elderly MSCs into four subpopulations by FACS. The original populations (young and elderly MSCs), as well as the four subpopulations, were then characterized before and after culture on tissue culture plastic and BM-derived extracellular matrix (BM-ECM). The small SSEA-4-positive subpopulation representing ~ 8% of the original elderly MSC population exhibited a "youthful" phenotype that was similar to that of young MSCs. The biological activity of this elderly subpopulation was inhibited by senescence-associated factors produced by the unfractionated parent population. After these "youthful" cells were isolated and expanded (three passages) on a "young microenvironment" (i.e., BM-ECM produced by BM cells from young donors), the number of cells increased ≈ 17,000-fold to 3 × 109 cells and retained their "youthful" phenotype.
Conclusions: These results suggest that it is feasible to obtain large numbers of high-quality autologous MSCs from the elderly population and establish personal stem cell banks that will allow serial infusions of "rejuvenated" MSCs for treating age-related diseases.
Keywords: Aging; Cellular senescence; Extracellular matrix; Senescence-associated secretory phenotype; Stem cell markers; Stem cell niche.
Conflict of interest statement
Ethics approval and consent to participate
Bone marrow, obtained from young donors with informed consent and in compliance with all federal regulations, was purchased commercially from LONZA (Walkersville, MD, USA; see company website for additional IRB information:
Consent for publication
Not applicable.
Competing interests
X-DC is a board member and shareholder in StemBioSys, Inc. (San Antonio, TX, USA). TJB is currently an employee of StemBioSys, Inc. and receives a salary. X-DC, TJB, and MM are inventors on a pending patent application relating to the content of the manuscript. The remaining authors have no competing or financial interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Powell J. The power of global aging. Aging Int. 2010;35:1–14. doi: 10.1007/s12126-010-9051-6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

