Enteric glial-mediated enhancement of intestinal barrier integrity is compromised by morphine
- PMID: 29078884
- PMCID: PMC5708166
- DOI: 10.1016/j.jss.2017.05.099
Enteric glial-mediated enhancement of intestinal barrier integrity is compromised by morphine
Abstract
Background: The opioid epidemic is a growing concern, and emerging evidence suggests that morphine use may be associated with sepsis. Enteric glial cells (EGCs) are the most numerous cell type in the enteric nervous system and regulate gastrointestinal function through the production of trophic factors, including glial-derived neurotrophic factor (GDNF). We sought to determine the effect of morphine on enteric glia and hypothesized that morphine contributes to EGC dysfunction and increased gut permeability.
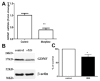
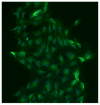
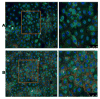
Materials and methods: Rat intestinal epithelial cells (IECs) and EGC lines were purchased from ATCC. Immunocytochemistry was used to evaluate the impact of EGCs on IEC barrier proteins and detect the μ-opioid receptor. Co-culture assays were used to determine the effect of EGCs, GDNF, and morphine on barrier integrity. Quantitative polymerase chain reaction and western blotting were performed to determine the impact of morphine in GDNF production. Transepithelial resistance of IEC-6 cell monolayers was measured in the presence of EGC-conditioned media (EGC-CM) and morphine treated EGC-CM using electrical cell impedance sensing.
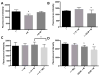
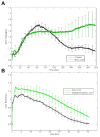
Results: EGC-CM enhanced tight junction organization in IECs. IEC barrier integrity was enhanced when co-cultured with unstimulated EGCs or with GDNF alone; this barrier protective effect was lost with morphine-treated EGCs. GDNF RNA and protein expression were decreased by morphine treatment. Transepithelial resistance was decreased in IEC confluent monolayers when exposed to morphine-treated EGC-CM compared with control.
Conclusions: Morphine compromises intestinal epithelial cell barrier function through a mechanism which appears to involve GDNF. Further studies are warranted to delineate the role of enteric glial cell function in opioid signaling and sepsis.
Keywords: Enteric glia; Enteric nervous system; GDNF; Intestinal barrier integrity; Morphine; Opioids.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures: The authors have no conflicts of interest or funding to disclose
Figures
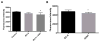
References
-
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(RR-1):1–49. DOI: http://dx.doi.org/10.15585/mmwr.rr6501e1. - DOI - PubMed
-
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, … Vallejo R. Opioid Complications and Side Effects. Pain Physician. 2008;11:S105–S120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

