Infection's Sweet Tooth: How Glycans Mediate Infection and Disease Susceptibility
- PMID: 29079498
- PMCID: PMC7125966
- DOI: 10.1016/j.tim.2017.09.011
Infection's Sweet Tooth: How Glycans Mediate Infection and Disease Susceptibility
Abstract
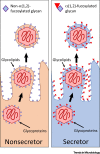
Glycans form a highly variable constituent of our mucosal surfaces and profoundly affect our susceptibility to infection and disease. The diversity and importance of these surface glycans can be seen in individuals who lack a functional copy of the fucosyltransferase gene, FUT2. Representing around one-fifth of the population, these individuals have an altered susceptibility to many bacterial and viral infections and diseases. The mediation of host-pathogen interactions by mucosal glycans, such as those added by FUT2, is poorly understood. We highlight, with specific examples, important mechanisms by which host glycans influence infection dynamics, including by: acting as pathogen receptors (or receptor-decoys), promoting microbial stability, altering the physical characteristics of mucus, and acting as immunological markers. We argue that the effect glycans have on infection dynamics has profound implications for many aspects of healthcare and policy, including clinical management, outbreak control, and vaccination policy.
Keywords: FUT2; glycosyltransferase; infection susceptibility; microbiota; viral infection.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
-
- Mathers C. World Health Organization; 2008. The Global Burden of Disease: 2004 Update.
-
- Liston A. Shaping variation in the human immune system. Trends Immunol. 2016;37:637–646. - PubMed
-
- McGuckin M.A. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011;9:265–278. - PubMed
-
- Thornton D.J. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008;70:459–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

