Inhibiting 12/15-lipoxygenase to treat acute stroke in permanent and tPA induced thrombolysis models
- PMID: 29079502
- PMCID: PMC5714685
- DOI: 10.1016/j.brainres.2017.10.024
Inhibiting 12/15-lipoxygenase to treat acute stroke in permanent and tPA induced thrombolysis models
Abstract
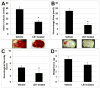
12/15-Lipoxygenase (12/15-LOX) contributes to the brain damage after middle cerebral artery occlusion (MCAO) in the acute phase of stroke. The aim of this study was to investigate the effects of a 12/15-LOX inhibitor, LOXBlock-1(LB1), in mice using a FeCl3-induced permanent distal MCAO model and FeCl3-induced ischemia/thrombolysis with tPA. In order to induce permanent distal MCAO, 30% FeCl3 was used in C57BL6 mice. LB1 or DMSO treatments were applied intraperitoneally 2 h following MCAO. For FeCl3-induced ischemia/thrombolysis experiments, 10% FeCl3 was preferred so as to obtain reperfusion with tPA in CD1 mice. 4 h following ischemia either LB1 or DMSO and iv tPA was administered. Outcomes were NSS, weight loss, infarct volume, hemorrhage area and reperfusion rate. FeCl3-induced distal MCAO caused an increase in 12/15-LOX signal in the ischemic cortex with an increase in MDA2 and AIF immunoreactivity. LB1 treatment, applied 2 h after ischemia, significantly decreased the infarct volume at 24 h of permanent distal MCAO. Weight loss was also significantly reduced in LB1 treated group. Distal MCAO and tPA application with LB1 or DMSO showed that treatment significantly decreased the infarct volume and the hemorrhage area. The reperfusion rate in the LB1-treated group was surprisingly higher than in the DMSO group and NSS results were significantly improved. These data suggest that LB1 can be used as an adjuvant agent to tPA. This study not only shows the effects of LB1 treatment in distal MCAO but also confirms that FeCl3-induced MCAO model can be a useful tool to screen novel treatment options in stroke.
Keywords: 12/15-LOX; FeCl(3); Reperfusion; Stroke models; tPA.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest: The authors declare that they have no conflict of interest.
Figures


References
-
- Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J Neurotrauma. 1996;13:557–68. - PubMed
-
- Denorme F, Langhauser F, Desender L, Vandenbulcke A, Rottensteiner H, Plaimauer B, Francois O, Andersson T, Deckmyn H, Scheiflinger F, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. 2016;127:2337–45. - PubMed
-
- Garcia-Yebenes I, Sobrado M, Zarruk JG, Castellanos M, Perez de la Ossa N, Davalos A, Serena J, Lizasoain I, Moro MA. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. - PubMed
-
- Hardingham GE, Lipton SA. Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid Redox Signal. 2011;14:1421–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

