A pivotal role for a conserved bulky residue at the α1-helix of the αI integrin domain in ligand binding
- PMID: 29079572
- PMCID: PMC5733610
- DOI: 10.1074/jbc.M117.790519
A pivotal role for a conserved bulky residue at the α1-helix of the αI integrin domain in ligand binding
Abstract
The ligand-binding βI and αI domains of integrin are the best-studied von Willebrand factor A domains undergoing significant conformational changes for affinity regulation. In both βI and αI domains, the α1- and α7-helixes work in concert to shift the metal-ion-dependent adhesion site between the resting and active states. An absolutely conserved Gly in the middle of the α1-helix of βI helps maintain the resting βI conformation, whereas the homologous position in the αI α1-helix contains a conserved Phe. A functional role of this Phe is structurally unpredictable. Using αLβ2 integrin as a model, we found that the residue volume at the Phe position in the α1-helix is critical for αLβ2 activation because trimming the Phe by small amino acid substitutions abolished αLβ2 binding with soluble and immobilized intercellular cell adhesion molecule 1. Similar results were obtained for αMβ2 integrin. Our experimental and molecular dynamics simulation data suggested that the bulky Phe acts as a pawl that stabilizes the downward ratchet-like movement of β6-α7 loop and α7-helix, required for high-affinity ligand binding. This mechanism may apply to other von Willebrand factor A domains undergoing large conformational changes. We further demonstrated that the conformational cross-talk between αL αI and β2 βI could be uncoupled because the β2 extension and headpiece opening could occur independently of the αI activation. Reciprocally, the αI activation does not inevitably lead to the conformational changes of the β2 subunit. Such loose linkage between the αI and βI is attributed to the αI flexibility and could accommodate the αLβ2-mediated rolling adhesion of leukocytes.
Keywords: VWA domain; cell adhesion; cell migration; integrin; leukocyte; von Willebrand factor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


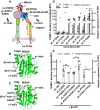
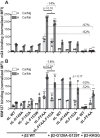
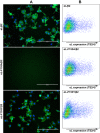

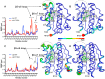

References
-
- Hynes R. O. (2002) Integrins: bi-directional, allosteric, signalling machines. Cell 110, 673–687 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

