Structural and functional effects of cytochrome b5 interactions with human cytochrome P450 enzymes
- PMID: 29079577
- PMCID: PMC5743060
- DOI: 10.1074/jbc.RA117.000220
Structural and functional effects of cytochrome b5 interactions with human cytochrome P450 enzymes
Abstract
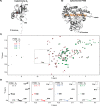
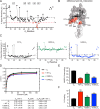
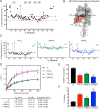
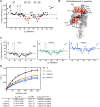
The small heme-containing protein cytochrome b5 can facilitate, inhibit, or have no effect on cytochrome P450 catalysis, often in a P450-dependent and substrate-dependent manner that is not well understood. Herein, solution NMR was used to identify b5 residues interacting with different human drug-metabolizing P450 enzymes. NMR results revealed that P450 enzymes bound to either b5 α4-5 (CYP2A6 and CYP2E1) or this region and α2-3 (CYP2D6 and CYP3A4) and suggested variation in the affinity for b5 Mutations of key b5 residues suggest not only that different b5 surfaces are responsible for binding different P450 enzymes, but that these different complexes are relevant to the observed effects on P450 catalysis.
Keywords: cytochrome; cytochrome P450; membrane protein; nuclear magnetic resonance (NMR); protein-protein interaction.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Waskell L., and Kim J.-J. P. (2015) Electron transfer partners of cytochrome P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th Ed (Ortiz de Montellano P. R., ed) pp. 33–68, Springer International Publishing, Cham, Switzerland
-
- Schenkman J. B., and Jansson I. (2003) The many roles of cytochrome b5. Pharmacol. Ther. 97, 139–152 - PubMed
-
- Bridges A., Gruenke L., Chang Y. T., Vakser I. A., Loew G., and Waskell L. (1998) Identification of the binding site on cytochrome P450 2B4 for cytochrome b5 and cytochrome P450 reductase. J. Biol. Chem. 273, 17036–17049 - PubMed
-
- Naffin-Olivos J. L., and Auchus R. J. (2006) Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry 45, 755–762 - PubMed
-
- Tamburini P. P., White R. E., and Schenkman J. B. (1985) Chemical characterization of protein-protein interactions between cytochrome P-450 and cytochrome b5. J. Biol. Chem. 260, 4007–4015 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

