c-MPL provides tumor-targeted T-cell receptor-transgenic T cells with costimulation and cytokine signals
- PMID: 29079582
- PMCID: PMC5746163
- DOI: 10.1182/blood-2017-02-769463
c-MPL provides tumor-targeted T-cell receptor-transgenic T cells with costimulation and cytokine signals
Abstract
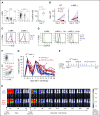
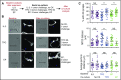
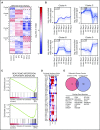
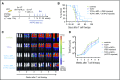
Adoptively transferred T-cell receptor (TCR)-engineered T cells depend on host-derived costimulation and cytokine signals for their full and sustained activation. However, in patients with cancer, both signals are frequently impaired. Hence, we developed a novel strategy that combines both essential signals in 1 transgene by expressing the nonlymphoid hematopoietic growth factor receptor c-MPL (myeloproliferative leukemia), the receptor for thrombopoietin (TPO), in T cells. c-MPL signaling activates pathways shared with conventional costimulatory and cytokine receptor signaling. Thus, we hypothesized that host-derived TPO, present in the tumor microenvironment, or pharmacological c-MPL agonists approved by the US Food and Drug Administration could deliver both signals to c-MPL-engineered TCR-transgenic T cells. We found that c-MPL+ polyclonal T cells expand and proliferate in response to TPO, and persist longer after adoptive transfer in immunodeficient human TPO-transgenic mice. In TCR-transgenic T cells, c-MPL activation enhances antitumor function, T-cell expansion, and cytokine production and preserves a central memory phenotype. c-MPL signaling also enables sequential tumor cell killing, enhances the formation of effective immune synapses, and improves antileukemic activity in vivo in a leukemia xenograft model. We identify the type 1 interferon pathway as a molecular mechanism by which c-MPL mediates immune stimulation in T cells. In conclusion, we present a novel immunotherapeutic strategy using c-MPL-enhanced transgenic T cells responding to either endogenously produced TPO (a microenvironment factor in hematologic malignancies) or c-MPL-targeted pharmacological agents.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.Y.L. receives royalty payments from Syros Pharmaceuticals and has sponsored travel from Novartis Institute for Biomedical Research; C.A. receives royalty payments from Cell Medica. The remaining authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

