Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies
- PMID: 29079599
- PMCID: PMC5777198
- DOI: 10.3324/haematol.2017.176917
Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies
Abstract
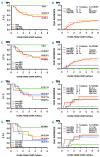
The prognostic value of minimal residual disease (MRD) in Philadelphia-chromosome-positive (Ph+) childhood acute lymphoblastic leukemia (ALL) treated with tyrosine kinase inhibitors is not fully established. We detected MRD by real-time quantitative polymerase chain reaction (RQ-PCR) of rearranged immunoglobulin/T-cell receptor genes (IG/TR) and/or BCR/ABL1 fusion transcript to investigate its predictive value in patients receiving Berlin-Frankfurt-Münster (BFM) high-risk (HR) therapy and post-induction intermittent imatinib (the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia (EsPhALL) study). MRD was monitored after induction (time point (TP)1), consolidation Phase IB (TP2), HR Blocks, reinductions, and at the end of therapy. MRD negativity progressively increased over time, both by IG/TR and BCR/ABL1. Of 90 patients with IG/TR MRD at TP1, nine were negative and none relapsed, while 11 with MRD<5×10-4 and 70 with MRD≥5×10-4 had a comparable 5-year cumulative incidence of relapse of 36.4 (15.4) and 35.2 (5.9), respectively. Patients who achieved MRD negativity at TP2 had a low relapse risk (5-yr cumulative incidence of relapse (CIR)=14.3[9.8]), whereas those who attained MRD negativity at a later date showed higher CIR, comparable to patients with positive MRD at any level. BCR/ABL1 MRD negative patients at TP1 had a relapse risk similar to those who were IG/TR MRD negative (1/8 relapses). The overall concordance between the two methods is 69%, with significantly higher positivity by BCR/ABL1. In conclusion, MRD monitoring by both methods may be functional not only for measuring response but also for guiding biological studies aimed at investigating causes for discrepancies, although from our data IG/TR MRD monitoring appears to be more reliable. Early MRD negativity is highly predictive of favorable outcome. The earlier MRD negativity is achieved, the better the prognosis.
Copyright© 2018 Ferrata Storti Foundation.
Figures




Comment in
-
Remission is good - relapse is bad.Haematologica. 2018 Jan;103(1):4-5. doi: 10.3324/haematol.2017.182667. Haematologica. 2018. PMID: 29290629 Free PMC article. No abstract available.
References
-
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. - PubMed
-
- Schrappe M, Camitta B, Pui CH, et al. Long-term results of large prospective trials in childhood acute lymphoblastic leukemia. Leukemia. 2000;14(12):2193–2320. - PubMed
-
- Aricò M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342(14):998–1006. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

