Treatment of essential thrombocythemia in Europe: a prospective long-term observational study of 3649 high-risk patients in the Evaluation of Anagrelide Efficacy and Long-term Safety study
- PMID: 29079600
- PMCID: PMC5777190
- DOI: 10.3324/haematol.2017.174672
Treatment of essential thrombocythemia in Europe: a prospective long-term observational study of 3649 high-risk patients in the Evaluation of Anagrelide Efficacy and Long-term Safety study
Abstract
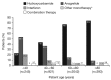
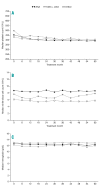
Evaluation of Anagrelide (Xagrid®) Efficacy and Long-term Safety, a phase IV, prospective, non-interventional study performed in 13 European countries enrolled high-risk essential thrombocythemia patients treated with cytoreductive therapy. The primary objectives were safety and pregnancy outcomes. Of 3721 registered patients, 3649 received cytoreductive therapy. At registration, 3611 were receiving: anagrelide (Xagrid®) (n=804), other cytoreductive therapy (n=2666), or anagrelide + other cytoreductive therapy (n=141). The median age was 56 vs. 70 years for anagrelide vs. other cytoreductive therapy. Event rates (patients with events/100 patient-years) were 1.62 vs. 2.06 for total thrombosis and 0.15 vs. 0.53 for venous thrombosis. Anagrelide was more commonly associated with hemorrhage (0.89 vs. 0.43), especially with anti-aggregatory therapy (1.35 vs. 0.33) and myelofibrosis (1.04 vs. 0.30). Other cytoreductive therapies were more associated with acute leukemia (0.28 vs. 0.07) and other malignancies (1.29 vs. 0.44). Post hoc multivariate analyses identified increased risk for thrombosis with prior thrombohemorrhagic events, age ≥65, cardiovascular risk factors, or hypertension. Risk factors for transformation were prior thrombohemorrhagic events, age ≥65, time since diagnosis, and platelet count increase. Safety analysis reflected published data, and no new safety concerns for anagrelide were found. Live births occurred in 41/54 pregnancies (76%). clinicaltrials.gov Identifier: 00567502.
Trial registration: ClinicalTrials.gov NCT00567502.
Copyright© 2018 Ferrata Storti Foundation.
Figures
References
-
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haemopoietic and lymphoid tissues, 2nd edition. 4th ed. Lyon: IARC Press; 2008.
-
- Xagrid Summary of Product Characteristics, Shire Pharmaceuticals Ltd; 2014. (Available from; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... Last accessed 9 February 2017)
-
- Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332(17):1132–1136. - PubMed
-
- Spivak JL, Hasselbalch H. Hydroxycarbamide: a user’s guide for chronic myeloproliferative disorders. Expert Rev Anticancer Ther. 2011; 11(3):403–414. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

