Consensus Guideline for Use of Glucarpidase in Patients with High-Dose Methotrexate Induced Acute Kidney Injury and Delayed Methotrexate Clearance
- PMID: 29079637
- PMCID: PMC5759822
- DOI: 10.1634/theoncologist.2017-0243
Consensus Guideline for Use of Glucarpidase in Patients with High-Dose Methotrexate Induced Acute Kidney Injury and Delayed Methotrexate Clearance
Abstract
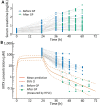
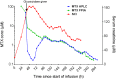
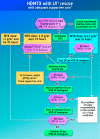
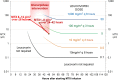
Acute kidney injury due to high-dose methotrexate (HDMTX) is a serious, life-threatening toxicity that can occur in pediatric and adult patients. Glucarpidase is a treatment approved by the Food and Drug Administration for high methotrexate concentrations in the context of kidney dysfunction, but the guidelines for when to use it are unclear. An expert panel was convened to provide specific, expert consensus guidelines for the use of glucarpidase in patients who develop HDMTX-induced nephrotoxicity and delayed methotrexate excretion. The guideline provides recommendations to identify the population of patients who would benefit from glucarpidase rescue by more precisely defining the absolute methotrexate concentrations associated with risk for severe or life-threatening toxicity at several time points after the start of an HDMTX infusion. For an HDMTX infusion ≤24 hours, if the 36-hour concentration is above 30 µM, 42-hour concentration is above 10 µM, or 48-hour concentration is above 5 µM and the serum creatinine is significantly elevated relative to the baseline measurement (indicative of HDMTX-induced acute kidney injury), glucarpidase may be indicated. After a 36- to 42-hour HDMTX infusion, glucarpidase may be indicated when the 48-hour methotrexate concentration is above 5 µM. Administration of glucarpidase should optimally occur within 48-60 hours from the start of the HDMTX infusion, because life-threatening toxicities may not be preventable beyond this time point.
Implications for practice: Glucarpidase is a rarely used medication that is less effective when given after more than 60 hours of exposure to high-dose methotrexate, so predicting early which patients will need it is imperative. There are no currently available consensus guidelines for the use of this medication. The indication on the label does not give specific methotrexate concentrations above which it should be used. An international group of experts was convened to develop a consensus guideline that was specific and evidence-based to identify the population of patients who would benefit from glucarpidase.
Keywords: Acute kidney injury; Creatinine; Glucarpidase; Leucovorin; Methotrexate.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Green MR, Chamberlain MC. Renal dysfunction during and after high‐dose methotrexate. Cancer Chemother Pharmacol 2009;63:599–604. - PubMed
-
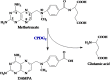
- Goldman P, Levy CC. The enzymatic hydrolysis of folate analogues. Biochem Pharmacol 1968;17:2265–2270. - PubMed
-
- Levy CC, Goldman P. The enzymatic hydrolysis of methotrexate and folic acid. J Biol Chem 1967;242:2933–2938. - PubMed
-
- Widemann BC, Sung E, Anderson L et al. Pharmacokinetics and metabolism of the methotrexate metabolite 2, 4‐diamino‐n 10‐methylpteroic acid. J Pharmacol Exp Ther 2000;294:894–901. - PubMed
-
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. The Oncologist 2006;11:694–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

