Novel Insights into the Pathogenesis of Monogenic Congenital Anomalies of the Kidney and Urinary Tract
- PMID: 29079659
- PMCID: PMC5748921
- DOI: 10.1681/ASN.2017050561
Novel Insights into the Pathogenesis of Monogenic Congenital Anomalies of the Kidney and Urinary Tract
Abstract
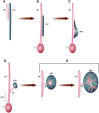
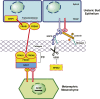
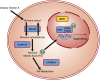
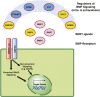
Congenital anomalies of the kidneys and urinary tract (CAKUT) comprise a large spectrum of congenital malformations ranging from severe manifestations, such as renal agenesis, to potentially milder conditions, such as vesicoureteral reflux. CAKUT causes approximately 40% of ESRD that manifests within the first three decades of life. Several lines of evidence indicate that CAKUT is often caused by recessive or dominant mutations in single (monogenic) genes. To date, approximately 40 monogenic genes are known to cause CAKUT if mutated, explaining 5%-20% of patients. However, hundreds of different monogenic CAKUT genes probably exist. The discovery of novel CAKUT-causing genes remains challenging because of this pronounced heterogeneity, variable expressivity, and incomplete penetrance. We here give an overview of known genetic causes for human CAKUT and shed light on distinct renal morphogenetic pathways that were identified as relevant for CAKUT in mice and humans.
Keywords: CAKUT; congenital anomalies of the kidneys and urinary tract; genetic kidney disease; monogenic disease.
Copyright © 2018 by the American Society of Nephrology.
Figures
References
-
- Chesnaye N, Bonthuis M, Schaefer F, Groothoff JW, Verrina E, Heaf JG, Jankauskiene A, Lukosiene V, Molchanova EA, Mota C, Peco-Antić A, Ratsch IM, Bjerre A, Roussinov DL, Sukalo A, Topaloglu R, Van Hoeck K, Zagozdzon I, Jager KJ, Van Stralen KJ; ESPN/ERA–EDTA registry : Demographics of paediatric renal replacement therapy in Europe: A report of the ESPN/ERA-EDTA registry. Pediatr Nephrol 29: 2403–2410, 2014 - PubMed
-
- (NAPRTCS) NAPRTCS : NAPRTCS 2008 Annual Report, Rockville, MD, The EMMES Corporation, 2008
-
- Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV: Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11: 720–731, 2015 - PubMed
-
- Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 - PubMed
-
- Loane M, Dolk H, Kelly A, Teljeur C, Greenlees R, Densem J; EUROCAT Working Group : Paper 4: EUROCAT statistical monitoring: Identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 91[Suppl 1]: S31–S43, 2011 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

