Nanog-driven cell-reprogramming and self-renewal maintenance in Ptch1 +/- granule cell precursors after radiation injury
- PMID: 29079783
- PMCID: PMC5660207
- DOI: 10.1038/s41598-017-14506-6
Nanog-driven cell-reprogramming and self-renewal maintenance in Ptch1 +/- granule cell precursors after radiation injury
Abstract
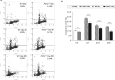
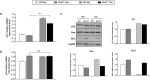
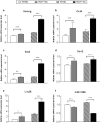
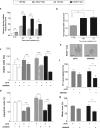
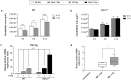
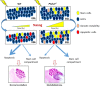
Medulloblastoma (MB) is the most common pediatric brain tumor, comprising four distinct molecular variants, one of which characterized by activation of the Sonic Hedgehog (SHH) pathway, driving 25-30% of sporadic MB. SHH-dependent MBs arise from granule cell precursors (GCPs), are fatal in 40-70% of cases and radioresistance strongly contributes to poor prognosis and tumor recurrence. Patched1 heterozygous (Ptch1 +/-) mice, carrying a germ-line heterozygous inactivating mutation in the Ptch1 gene, the Shh receptor and negative regulator of the pathway, are uniquely susceptible to MB development after radiation damage in neonatal cerebellum. Here, we irradiated ex-vivo GCPs isolated from cerebella of neonatal WT and Ptch1 +/- mice. Our results highlight a less differentiated status of Ptch1-mutated cells after irradiation, influencing DNA damage response. Increased expression levels of pluripotency genes Nanog, Oct4 and Sal4, together with greater clonogenic potential, clearly suggest that radiation induces expansion of the stem-like cell compartment through cell-reprogramming and self-renewal maintenance, and that this mechanism is strongly dependent on Nanog. These results contribute to clarify the molecular mechanisms that control radiation-induced Shh-mediated tumorigenesis and may suggest Nanog as a potential target to inhibit for adjuvant radiotherapy in treatment of SHH-dependent MB.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

